Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Diiron Enzymes Spark Hydroxylations
Diiron monooxygenases join monoiron enzymes in the ability to perform β-hydroxylation of amino acids
by Jyllian N. Kemsley
August 23, 2010
| A version of this story appeared in
Volume 88, Issue 34

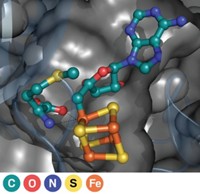
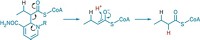
Monooxygenase enzymes incorporating an oxygen-bridged diiron cluster in the catalytic site represent a new class of enzymes that perform β-hydroxylation of amino acids, reports a research group led by Thomas M. Makris and John D. Lipscomb of the University of Minnesota, Minneapolis (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1007953107). In natural products used as chemotherapeutic and antimicrobial agents, hydroxylation at the β-carbon of amino acids is common. These hydroxylations were previously known to be catalyzed only by monoiron α-ketoglutarate-dependent dioxygenases or cytochrome P450 monooxygenases. Lipscomb’s group hypothesized that diiron enzymes could catalyze the same reaction, and they found that enzymes in several biosynthetic pathways had sequences that were consistent with the presence of dinuclear metal sites. Detailed studies of CmlA, an enzyme that facilitates the β-hydroxylation step in the biosynthesis of the antibiotic chloramphenicol, revealed that the enzyme indeed contains a diiron center and has a structure previously unseen in monooxygenases. A better understanding of these enzymes and their mechanism will be important in efforts to develop nonnatural products through biosynthetic engineering, says biochemist Jason Micklefield of the University of Manchester, in England.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter