Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Megaenzyme Crystal Structure Unveiled
The X-ray structure of a 750-kilodalton bacterial carboxylase should provide clues about human diseases
by Celia Henry Arnaud
August 23, 2010
| A version of this story appeared in
Volume 88, Issue 34

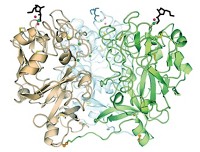
A team led by Liang Tong of Columbia University is reporting a 3.2-Å X-ray crystal structure of the 750-kilodalton bacterial enzyme propionyl-coenzyme A carboxylase (PCC), a feat that is anticipated to provide clues about human diseases (Nature 2010, 466, 1001). The bacterial protein consists of six α subunits containing the biotin carboxylase (BC) and biotin carboxyl carrier protein domains and six β subunits containing the carboxyltransferase (CT) domain. The crystal structure reveals that the enzyme consists of a central, cylindrical β6 hexamer core decorated with three α subunits at both ends. The BC and CT active sites are separated by about 55 Å, and a previously unknown domain in the α subunits mediates interactions between them. Cryoelectron microscopy studies at 15-Å resolution suggest that human PCC has a structure similar to that of the bacterial enzyme. The structure could help uncover the molecular basis of disease-causing mutations in human PCC, the researchers note. For example, deficiencies in the human version are associated with propionic acidemia, a potentially fatal recessive genetic disorder. The structure could also provide insight into other biotin-dependent carboxylases, they add.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter