Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Binding Site Broadens Prospects For Prostate-Cancer Drugs
Scientists have found a new binding site in prostate-specific membrane antigen, a cancer-cell-surface receptor
by Stuart A. Borman
September 6, 2010
| A version of this story appeared in
Volume 88, Issue 36

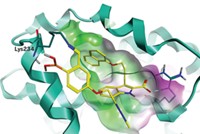
In work that could aid the search for prostate cancer treatments, scientists have identified a new binding site in a cell-surface receptor that has become an important target for prostate cancer drugs. Last year, David A. Spiegel of Yale University and coworkers developed a class of small molecules called ARM-Ps (antibody-recruiting molecules targeting prostate cancer) that kill prostate cancer cells by a novel mechanism: They bind to cytotoxic antibodies and deliver them to prostate-specific membrane antigen (PSMA), a receptor on prostate cancer cell surfaces. Now, in a study of ARM-P analogs, Spiegel’s group has found a previously unreported arene-binding site in PSMA that improves binding affinities (J. Am. Chem. Soc., DOI: 10.1021/ja104591m). ARM-P derivatives that hit the new site have affinities as low as 20 pM, among the best reported affinities for PSMA. In addition to the site’s therapeutic implications, Spiegel says he believes its structural simplicity—it is composed of only three amino acids—“suggests the intriguing possibility that such sites are broadly present in the proteome and could serve as useful tools in the optimization of protein-ligand interactions in general.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter