Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Technique Combo Beats NMR Solo
Structural Biology: Combined strategy yields largest-ever solution structures
by Stu Borman
September 6, 2010
| A version of this story appeared in
Volume 88, Issue 36

Researchers have combined nuclear magnetic resonance (NMR) with X-ray and neutron scattering to solve the largest protein solution structures to date. The structures illuminate how a key bacterial signal transduction pathway works and could lead to new antibiotics. The approach could also be useful for structural analysis of other large biomolecules.
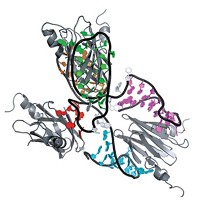
Marius Clore of the National Institutes of Health and coworkers obtained the structures of the 128-kilodalton enzyme I (EI) dimer and its 146-kDa complex with histidine phosphocarrier protein (HPr) (J. Am. Chem. Soc., DOI: 10.1021/ja105485b). EI and HPr catalyze initial steps in a phosphotransfer process that transports sugar across bacterial cell membranes.
Biomolecular solution structures in the 50- to 80-kDa range have been solved by NMR alone, but the new study extends the accessible size range significantly by using multiple complementary solution techniques.
Crystal structures of EI had been solved previously, but they disagree with solution scattering data, indicating that domain orientations in crystals and in solution differ. The combined approach resolves these discrepancies and thus provides information that is not accessible from crystal structures alone.
In the combined technique, small- and wide-angle X-ray scattering provide shape and size information, NMR dipolar couplings (a type of internuclear interaction) indicate how to orient the domains properly, and small-angle neutron scattering validates the structures.
Other groups have used NMR and scattering techniques together, but Clore’s is the “first to demonstrate the combined approach experimentally on a biologically relevant high-molecular-weight complex,” says structural biologist Michael Sattler of the Technical University of Munich and Helmholtz Center Munich. And although complexes of similar size have been structurally analyzed, those studies were qualitative or focused on individual regions, whereas the Clore study “provides a full experimental description of a complete complex. It’s an impressive demonstration of the utility and success of hybrid approaches for structural analysis of challenging systems,” Sattler says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter