Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Raman Heads For The Clinic
Vibrational spectroscopy technique may someday be used to diagnose a variety of diseases
by Celia Henry Arnaud
September 20, 2010
| A version of this story appeared in
Volume 88, Issue 38

Disease diagnosis is still something of an art. Like many arts, it involves a healthy dose of subjectivity. Methods that provide objective information could help doctors make better, faster diagnoses. Raman spectroscopists hope that they can provide one of those methods.
At last month’s International Conference on Raman Spectroscopy (ICORS), researchers from around the world described their work to use Raman to diagnose a variety of diseases. Some have already performed small-scale clinical studies. For others, such studies are imminent. In still other cases, clinical application is further away, but the end goal is clearly in sight. The most advanced of these medical applications of Raman could be ready for the market in less than five years.
Raman has both strengths and weaknesses for medical applications. As a type of vibrational spectroscopy, it can take advantage of intrinsic molecular differences between various chemicals in the body to draw pictures that traditionally require the introduction of tags or stains. Thus it can eliminate the need for some sample preparation and can reduce the possibility of perturbing the system. However, the most traditional form of Raman—spontaneous Raman scattering—scatters only a tiny fraction of incident photons at a frequency different from the frequency of the incident light. The weakness of this effect means that Raman is not particularly sensitive.
One application that Raman is particularly suited to is bone analysis, such as that required to track the treatment of osteoporosis. The mineral components of bone produce strong, easily distinguishable Raman bands. Bone consists of mineral, primarily hydroxyapatite, over a protein matrix composed mainly of collagen. The Raman spectra reveal the quality of the bone tissue, and the spectra vary with a number of factors, including age, exercise, diet, mechanical loading, damage, and disease, says Michael D. Morris, a chemistry professor at the University of Michigan who has been studying bones with Raman for more than a decade.
One of the challenges in acquiring Raman spectra of bone is the presence of other tissues surrounding the bone, including skin, subcutaneous fat, ligaments, and muscles. Using a technique called spatially offset Raman spectroscopy, in which the scattered light is collected from a different spot than the incident light, Morris can acquire Raman spectra from tissue that is far below the surface.
Even in the presence of these other tissues, the signal from the mineral is easy to pick out in these spectra, Morris says. “You’re looking for a needle in a haystack, but this is the only needle made of hydroxyapatite.”
Morris hopes to use Raman in osteoporosis studies to predict the risk of fractures and assess the effectiveness of therapy. “The diagnosis of osteoporosis is not nearly as important as following up on therapy,” he says. “Basically, demography is a pretty good diagnosis,” meaning that generally speaking, small-boned women over a certain age are at high risk of osteoporosis.
Therapeutic progress in osteoporosis is generally tracked with a method called dual-energy X-ray absorptiometry, or DXA, in which bone density is measured. But DXA is poorly predictive of therapeutic outcomes, Morris says. In contrast, the mineral composition and quality, measured with Raman, provide “quite a bit of predictive power,” Morris says.
Even after Morris and his colleagues begin using Raman to predict outcomes for osteoporosis patients, they won’t know whether it’s effective for at least three years. The disease can take years to progress, and it can take years to determine whether treatment has worked. As a result, “to the horror of physical chemists, we don’t get an answer in an afternoon or even days or weeks. We get it in years.”
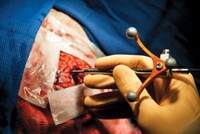
Morris and his collaborators have made measurements of bone in cadaver limbs, but they have yet to demonstrate their method with living patients. They hope to soon begin a trial in which they will use fiber-optic probes to directly measure Raman spectra of bone in patients undergoing knee surgery, to validate their interpretation of spectra through layers of tissue.
Morris hopes that Raman will prove to be a valuable adjunct to other diagnostic techniques currently in use. “Ultimately, nothing is stand-alone,” he says. “We think the composition information that we provide does actually have predictive value. Three years from now,” the amount of time necessary for a forward-looking trial of osteoporosis, “I’ll either be very humble or very proud,” he says.

A related application of Raman is the detection of early dental caries, more commonly known as tooth decay. Lin-P’ing Choo-Smith, a researcher at the National Research Council Canada’s Institute for Biodiagnostics, in Winnipeg, Manitoba, is developing Raman probes to look at structural changes involved in tooth decay with the goal of catching such decay before “drill and fill” becomes the only option.
As with bone, hydroxyapatite is the main mineral component of teeth. “There’s a big peak in the Raman spectrum that’s specific to that phosphate,” Choo-Smith says. “The phosphate peak tells us information about the mineral quality and content of the tooth.” In the process of tooth decay, acid produced primarily by bacteria in dental plaque leaches the minerals from teeth.
The Raman approach is appropriate for early-stage lesions that are about 100- to 250-μm deep, Choo-Smith says. At these early stages, clinicians have multiple options, including treatment with fluoride, sealants, or antimicrobials. Although Raman could also be used for more advanced cavitated stages, “there’s really no point, because the clinician can already see it very clearly” visually or in X-rays, Choo-Smith says.
In their first Raman spectra of extracted teeth, the spectral differences between sound and carious enamel were too subtle to allow fast spectral acquisition, Choo-Smith says. When they instead tried polarized Raman, in which polarized light is used to acquire the spectrum, they saw much larger changes between sound and carious enamel. Now, they use the depolarization ratio—the ratio of the intensities in cross-polarized and parallel-polarized Raman spectra—as their metric. She expects that they will use the polarization ratio to construct a scale that correlates with the extent of tooth decay and suggested treatments.
In laboratory experiments, Choo-Smith and her collaborators have observed spectral changes that occur in response to demineralization and remineralization. Demineralization involves intensity changes, whereas remineralization results in frequency shifts that reflect the mineral quality and crystallinity of the repaired structure.
This month, Choo-Smith expects to begin working with actual patients. “We’ve gone through all the ethics approval,” she says. “We’re just waiting for some final approvals to do it in vivo. We’re very close to trying it in people.”
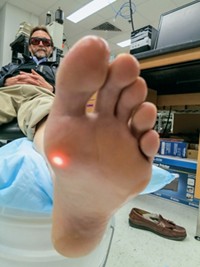
Another potential clinical application for Raman is noninvasive profiling of metabolites in blood, an application perhaps more frequently associated with near-infrared absorption or diffuse reflectance spectroscopy. Joseph Chaiken of Syracuse University is working with the company LighTouch Medical to develop Raman methods for sensing glucose and other analytes. Because of Raman’s weak signals, the methods will be limited to small-molecule species, such as glucose, that are present at millimolar concentrations or large molecules, such as proteins, that occur at lower concentrations but have repeating bonds that result in a “piling up” of Raman features. Analytes in blood that might be amenable to noninvasive Raman include hemoglobin, bicarbonate, cholesterol, triglycerides, urea, lactic acid, total protein (but not individual proteins), and glucose.
Chaiken and coworkers use modulation techniques to pick out the signal for an analyte of interest from interfering signals in blood and surrounding tissues. In particular, they use pressure to change the distribution of blood in tissue relative to the static background tissue, such as skin, and then subtract the spectrum acquired with applied pressure from the spectrum without applied pressure.
Chaiken suspects that noninvasive Raman measurements will be restricted to spontaneous Raman or at most weakly resonance-enhanced Raman rather than strongly resonance-enhanced Raman, which would be more sensitive. “To exploit significant resonance enhancement, there has to be significant absorption” of incident light, he says. “If there’s too much absorption, there’s excessive energy deposition, and you get what we call a self-cauterizing wound,” he explains.
At ICORS, Chaiken presented noninvasive, in vivo spectra of hemoglobin. This protein makes an excellent model system because its spectrum is well established in vitro and it is weakly resonance enhanced with the near-infrared excitation that is required for in vivo applications. “I wanted people to see that we could unambiguously show you something that was being measured inside the body without taking any blood out,” Chaiken says. It’s harder to make the case that you can detect glucose with Raman because its spectrum is not nearly as strong or as distinct as that of hemoglobin, he adds. Nevertheless, Chaiken points out that his group and two other groups have independently claimed over the past 10 years that they can indeed measure glucose using noninvasive Raman.

Perhaps the most avidly sought targets for clinical Raman are the many forms of cancer. Researchers continue to search for a molecular signature that can be used to discriminate between healthy and diseased tissue, as well as between the various stages of disease. But because the Raman spectrum contains no explicit “cancer peak,” they are forced to rely on statistical analysis of spectra.
At ICORS, Zhiwei Huang, an assistant professor in the department of bioengineering at the National University of Singapore, reported on his group’s efforts to use Raman spectroscopy to diagnose stomach cancer in its earliest stages. Stomach cancer is the second-leading cause of cancer deaths worldwide, according to the World Health Organization.
Working with physicians at the university’s Yong Loo Lin School of Medicine, Huang and his coworkers used a specially designed Raman endoscopic probe to make in vivo tissue measurements. They used wide-field imaging to select regions for Raman analysis. From 30 patients, the researchers collected a total of 72 spectra, 54 of them from normal tissue and 18 from precancerous lesions, which were confirmed by conventional histopathology. Their data analysis method correctly identified 52 of the normal sites and 17 of the precancerous sites.
Another common cancer, particularly in the developing world, is cervical cancer. “Cervical cancer is extremely treatable and curable,” says Elizabeth A. Vargis, a graduate student in biomedical engineering in Anita Mahadevan-Jansen’s group at Vanderbilt University. “The problem is finding it.” The hope is that Raman could reduce the number of follow-up appointments, especially in the developing world, where women are less likely to return for follow-up, by immediately indicating the need for follow-up during the initial appointment, Vargis says.
Vargis and Mahadevan-Jansen are in the midst of a clinical trial in which they make Raman measurements of the cervix of women who are undergoing Pap smears, the standard diagnostic procedure for cervical cancer. They are also approved to make measurements of the cervix of women who are returning for a follow-up biopsy after an abnormal Pap smear. They have collected spectra from tissue that ended up being normal and that fell within three categories of abnormal: metaplastic and low- and high-grade dysplastic.
Using statistical analysis, the researchers are able to correctly classify 88% of the tissues. However, when they look at the value of the predictive probability for the correctly identified samples, the amount of scatter is unacceptable.
Suspecting that the Raman spectrum was picking up changes other than those simply caused by cancer, Vargis and Mahadevan-Jansen added other factors to their analysis, including the hormonal state of the patients. When they studied only premenopausal women, the classification accuracy improved to 94%, with much less scattering.
They continue to look for ways to improve prediction accuracy. “We decided to see if we could tease out anything else that could be affecting the Raman or affecting the cervix itself,” Vargis says. They have found that factors such as ethnicity, socioeconomic status, and body mass index could affect hormonal levels and thus the Raman spectra.
“Raman is a lot more sensitive than we thought,” Vargis says. “When we tried to look at the data more closely, we found it was picking up normal things we hadn’t considered.”
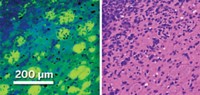
In addition to being used directly with patients, Raman can be used to analyze biopsy and surgical pathology samples for signs of cancer. Shona Stewart and coworkers at the Pittsburgh-based company ChemImage Corp. have collaborated with pathologists at Allegheny General Hospital to produce Raman images from kidney cancer biopsies that can be read just like conventionally stained tissue slices. They have shown that it is possible to use such images to distinguish benign cells from malignant ones.

Other projects have not yet advanced to the point of working with human subjects. For example, Pavel Matousek, a spectroscopist at the Rutherford Appleton Laboratory in Didcot, England; Nicholas Stone, head of the Biophotonics Research Unit at Gloucestershire Royal Hospital in Gloucester, England; and coworkers hope to use “deep” Raman to analyze calcifications in breast tissue that can be indicative of breast cancer. The composition of the calcifications, which consist primarily of hydroxyapatite, the same material found in bone and teeth, varies with disease state and can serve as a measure of malignancy. These calcifications are what usually show up in mammograms, and they indicate where biopsies should be taken. However, the vast majority of these lesions are benign, leading to unnecessary biopsies.
Advertisement
Using their deep Raman procedure, Matousek and Stone can collect spectra from several centimeters below the surface of breast tissue. They hope that their method will eventually be integrated with conventional mammography and provide composition information that could drastically reduce the number of unnecessary biopsies.
“We could target those calcifications and make a decision about whether they’re benign or malignant. If they’re malignant or look like they are, you would come back for a biopsy. If they’re benign, which is 80 to 90% of the cases, you would not come back for a biopsy,” Stone says. “In the U.K. alone, that would save about 80,000 patients from having secondary procedures.”
The carbonate composition of the calcification changes with the disease state. Benign calcifications have a higher carbonate concentration than do malignant ones, Stone says. The amount of carbonate affects the phosphate peak, which is much stronger than the carbonate peak, so Matousek and Stone simply measure the width and position of the phosphate peak.
They plan to use a system with fiber-optic probes. They don’t yet know, Stone says, whether they will just shine light through the compression plates used in mammography or whether they will thread the probe past the plates.
Whereas the other applications are based on spontaneous Raman, X. Sunney Xie and his collaborators are developing a nonlinear Raman spectroscopy called stimulated Raman scattering (C&EN, Dec. 22, 2008, page 6). One of their primary goals is to use this method as a replacement for the hematoxylin and eosin stains used in conventional pathology.
In many of the spontaneous Raman applications, the goal is to supplant the traditional pathology diagnosis. But Xie’s team asks, why not harness the accumulated knowledge of pathologists and give them images—constructed with Raman “stains”—that look like the ones they’re used to working with? Using Raman bands that are specific to lipids and proteins to construct images, Xie and his collaborators can slash the time needed for processing a tissue slice for conventional pathology.
Xie and Geoffrey S. Young, a radiologist at Harvard Medical School and Brigham & Women’s Hospital, in Boston, have been working with pathologists on animal models, but they believe that they are technologically ready to move to human systems.
“The pathologists on our team have given us very good feedback that by looking at these models, they can make the same types of diagnoses” they do with conventional hematoxylin and eosin stains, Young says.
He and Xie are particularly interested in using their method to generate diagnostic images during brain surgery. The 20-minute cycle required for conventional histopathology slows neurosurgery while surgeons wait to find out if they need to excise more tissue. In addition, the flash-freezing required for conventional pathology introduces artifacts: The freezing lyses the cells and they don’t stain well, Young says. For these reasons, images produced by stimulated Raman scattering “should actually be better in a lot of ways than the traditional histopathologic images,” Young says. “But obviously in the initial stages, the ultimate aim is to demonstrate that they’re equivalent.”
One of the hurdles to moving stimulated Raman scattering to the clinic is the fact that the technique requires two lasers, instead of the one that is needed for spontaneous Raman. Therefore, the instrument could be too large for some applications. Such problems could be easily solved, Xie notes, by the introduction of fiber lasers, which are much more compact than solid-state lasers.
At this point, no one knows what clinical Raman instruments will look like if and when they become commercialized. Will there be a generic instrument with specialized probes, or will there be a dedicated device for each application? The answer to that question depends on price.
“The more specialized you make it, the simpler you can make it, which means the less expensive and more foolproof you can make it,” Morris says. “The advantage of having a bunch of different probes is a lot of people can use it. If this has to be an expensive instrument, that’s clearly the way to go.”
Regardless of what the final systems look like, researchers are already showing that Raman has a bright future in the clinic.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter