Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Fetal Origins Of Disease
Efforts are under way to link chemical exposures in utero with adult disease
by Britt E. Erickson
November 8, 2010
| A version of this story appeared in
Volume 88, Issue 45

A growing body of scientific research suggests that exposure to chemical toxicants in the womb can lead to chronic health problems, including obesity, diabetes, heart disease, and cancer, later in life. Although scientists agree that the evidence is compelling, many of them are frustrated because such data aren’t being used in regulatory decision-making and risk assessment.
To help bridge the gap between emerging scientific research and environmental-health regulatory decisions, a standing committee of the National Academies held a two-day workshop last month in Washington, D.C. The committee, along with a handful of invited toxicologists and risk assessors, debated whether predicting adult disease from in utero and postnatal indicators is ready for prime-time risk assessment.
Researchers presented two case studies to demonstrate how science is emerging in this area. One case study examined fetal origins of obesity, insulin resistance, and high blood pressure, and another investigated adult diseases associated with in utero exposure to arsenic.
The scientists showed that under- and overnutrition, intrauterine growth restriction, and exposure to chemical toxicants and certain drugs in utero can lead to changes in gene expression, tissue function, and developmental pathways that increase susceptibility to various diseases later in life.
In both case studies, epigenetics—the processes that alter gene expression without changing the gene sequence—played a key role in the development of disease. Such processes include DNA methylation, histone modification, and the formation of microRNAs, all of which fine-tune gene expression to help prepare an organism for what it will encounter later in life.
“It has long been accepted that our genes together with adult lifestyle determine our phenotype and susceptibility to a whole range of chronic diseases,” noted Karen A. Lillycrop, a biochemist at the University of Southampton, in England. “But there is now increasing evidence that the prenatal and early postnatal environment also play a key role in determining our susceptibility to such diseases.”
Scientists at the workshop showed that epigenetic biomarkers can be used to predict future disease risk. Lillycrop, for example, presented evidence from both animal and human studies suggesting that DNA methylation marks at birth are correlated with susceptibility to obesity and various metabolic diseases later in life. She showed that such changes in DNA methylation can be induced by both under- and overnutrition.
Lillycrop also explained that this methylated DNA remained unchanged in human blood samples taken up to 20 years apart. “It is thought that these methylation marks are stable and maintained on our genes throughout our life,” she said. It may, however, be possible to prevent and reverse such epigenetic changes through interventions, such as folic acid supplementation, if such interventions are done early enough in life.
Other factors besides nutrition also are responsible for epigenetic changes. Bruce Blumberg, a developmental biologist at the University of California, Irvine, presented work from his lab showing that in utero exposure to obesogens—chemicals that stimulate fat storage—can lead to weight gain and obesity later in life. In particular, Blumberg showed that tributyltin binds to receptors that regulate hormonal control of fat cell development and lipid balance. The end result is increased expression of adipogenic pathway genes, increased stem cell differentiation into adipocytes, and obesity.
“Diet and exercise are insufficient to explain the obesity epidemic, particularly in six-month-old babies,” Blumberg emphasized. “Prenatal obesogen exposure reprograms exposed animals to be fat,” he said.
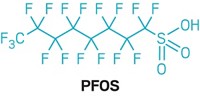
Many chemicals have been found to be obesogens, including prescription drugs and environmental contaminants, Blumberg noted. He pointed to data in the literature showing obesity correlated with exposure to the antidiabetic drugs Actos and Avandia, atypical antipsychotics, antidepressants, COX-2 inhibitors, environmental estrogens such as bisphenol A and perfluorinated octanoic acid, phthalates, nicotine, air pollution, benzo[a]pyrene, and fructose.

“What we don’t know is how many obesogens are out there,” Blumberg stressed. “Is there just this small handful, hundreds, thousands? We don’t know the answer. We don’t know what the body burden is in people either. We don’t know what all the modes of action are.”
In addition to obesity, the workshop explored fetal origins of other diseases such as heart disease and cancer. Several scientists presented data suggesting an association between these diseases and in utero exposure to arsenic.
Michael P. Waalkes, a researcher in the National Toxicology Program’s inorganic toxicology group, reported that fetal exposure to inorganic arsenic in drinking water alters stem cell numbers and their activity, leading to cancer in mice. And once the cancer stem cells form, they are permanent, Waalkes noted. He showed that arsenic exposure causes decreased expression of the PTEN tumor suppressor gene, leading to an overabundance of cancer stem cells.
Other epigenetic changes induced by arsenic exposure were revealed by J. Christopher States, a molecular biologist at the University of Louisville School of Medicine. States showed that fetal arsenic exposure accelerates and exacerbates atherogenesis—the formation of plaque in arteries—and leads to an increased risk of cardiovascular disease. He also showed that prenatal arsenic exposure leads to DNA methylation changes in the liver causing it to persistently overactivate inflammation pathways.
Similarly, Joseph H. Graziano, a professor of environmental health sciences and pharmacology at Columbia University, explored the consequences of prenatal and postnatal exposure to arsenic in Bangladesh, where high levels of arsenic naturally contaminate groundwater used as drinking water. He too discovered altered DNA methylation and gene expression induced by arsenic. The affected genes are involved in inflammation, cell signaling, stress response, and apoptosis.
In general, participants at the workshop agreed that the evidence linking early-life changes to disease outcomes in adults is pretty convincing. But many of them said more examples are needed before such science can be incorporated into regulatory decision-making and risk assessment.
“While it seems reasonable that these changes would drive the later effects, the certainty that these are the cardinal causative pathways needs bolstering,” said Robert E. Chapin, a reproductive toxicologist with Pfizer. “And would these pathways, if measured early, report all the obesogens and carcinogens? How many different ways are there to get to the final outcome?”
A similar point was brought up by Theodore Slotkin, a developmental neurotoxicologist at Duke University Medical Center. “The causes of obesity are many. Totally different mechanisms may have different epigenetic changes, and yet the disease outcome is the same,” he said. “There isn’t the time and money to connect every chemical to every possible outcome. From the point of view of the regulators, without causal mechanisms, we are right back at square one.”
Slotkin suggested that rather than looking at the most common outcomes, such as obesity and heart disease, researchers should look at the most uncommon outcomes. He pointed out that the connection between smoking and lung cancer was made in the 1930s “because lung cancer was a disease that never happened.”
Many workshop participants said that they are frustrated because their results aren’t being used in risk assessments. “What kind of data do we need to produce that will have the most impact in risk assessment?” Blumberg asked.
“For science to be useful, it has to answer specific questions that a risk manager wants to know about,” said Ila Cote, a senior science adviser at the Environmental Protection Agency’s National Center for Environmental Assessment. “What are the adverse effects, at what concentrations; are subpopulations affected; and how certain are the data?”
But Cote acknowledged that there are also social and political barriers to getting new science into risk assessments. “Lawyers would much rather you continue to do it the way you’ve always done it than to make a shift,” she said. “Both the vigorous discussions you can get in with stakeholders and this requirement that whatever your decisions are they have to stand up in court tend to make government scientists pretty darn conservative.”
Another problem has to do with getting funding for the type of work that regulatory agencies such as EPA find useful. For example, the National Institutes of Health rarely funds experiments that could actually help EPA and other agencies make decisions, said Bruce Fowler, a toxicologist at the Agency for Toxic Substances & Disease Registry. Unless grant reviewers start thinking about the practical outcomes of the data, researchers will continue to pursue basic scientific interests first, he pointed out.
The Food & Drug Administration is also interested in understanding the long-term effects of early-life chemical exposures because many drugs can interfere with developmental pathways, said Deborah K. Hansen, a research biologist with FDA’s National Center for Toxicological Research. But “if you intervene for one outcome, you can alter something else that may end up being adverse,” she cautioned.
It remains to be seen how many chemicals to which children are exposed in the womb are responsible for causing or increasing the susceptibility to disease later in life. And it is unlikely that regulatory agencies will use this emerging science in risk assessments any time soon. Nonetheless, despite their frustration, many scientists are optimistic that they are on the right path.
“The chance to set up a process that could actually help prevent many of our major current adulthood diseases and increase improved outcomes for all kids—that’s got to be seen as the most compelling opportunity we’ve ever had,” Chapin remarked. “This is not an issue of, ‘Should we do this?’ I think we’ve got to at least look and see how prevalent the effects are.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter