Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Halogenation In The Garden
Synthetic Biology: Chemists integrate carbon-halogen bond formation into plant metabolism
by Stephen K. Ritter
November 8, 2010
| A version of this story appeared in
Volume 88, Issue 45

By engineering a popular garden plant to express halogenase enzymes from soil bacteria, a team of Massachusetts Institute of Technology researchers has expanded the plant’s ability to biosynthesize complex natural products to include making rare halogenated analogs. The development could make it easier to produce desirable halogenated pharmaceuticals in plants rather than in engineered bacteria or by way of elaborate multistep chemical syntheses.
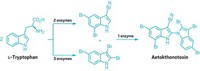
Weerawat Runguphan and Sarah E. O’Connor previously engineered the Madagascar periwinkle, Catharanthus roseus, to convert exogenous halogenated tryptamines into halogenated alkaloids. Runguphan, O’Connor, and Xudong Qu now report that they have engineered the plants to make the halogenated tryptamines themselves (Nature, DOI: 10.1038/nature09524).
The researchers overexpressed bacterial RebH or PyrH halogenase enzymes, which work with a partner reductase, RebF, to selectively chlorinate tryptophan in the 7-position or 5-position, respectively. The plant’s natural tryptophan decarboxylase enzyme takes over to convert the chlorotryptophan to chlorotryptamine, which is shuttled into the plant’s alkaloid biosynthetic pathway where it is fused with the monoterpene secologanin. This intermediate is further functionalized to yield various chlorinated alkaloids in plant cell cultures. With RebH, the process can also produce brominated analogs.
The report by O’Connor’s group “represents a crowning achievement to rationally engineer the biosynthesis of unnatural chlorinated alkaloids,” says Bradley S. Moore of Scripps Institution of Oceanography. “While this type of metabolic reengineering is now commonplace in microbial systems, it is unprecedented in more complex higher organisms.”
Earlier this year, Moore’s group collaborated with David O’Hagan’s group at Scotland’s University of St. Andrews to clone the first fluorinase gene from a soil bacterium into a marine bacterium to produce a fluorinated analog of the anticancer compound salinosporamide.
“We are in an exciting new phase in metabolite engineering,” O’Hagan says. “It is clear that the tools are developing to selectively halogenate natural products by biotechnological, rather than chemical, methods.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter