Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Spinning Both Ways
Nanotechnology: Researchers develop molecular motor that whirls in two directions
by Sarah Everts
November 2, 2010
| A version of this story appeared in
Volume 88, Issue 45

Using a flash of light, a bit of base, and principles of molecular chirality, researchers led by Ben L. Feringa of the University of Groningen, in the Netherlands, have designed a rotating molecular motor that can be instructed to spin forward or in reverse (Nature Chem., DOI: 10.1038/nchem.872).
"There are relatively few examples of artificial molecular machines in the literature where unidirectional motion has been demonstrated, let alone it being shown that it is possible to reverse the direction of motion," comments Fraser Stoddart, a chemist at Northwestern University. This "tour de force" advance could be used in "the construction of adaptive materials, wherein the function of some kind of integrated system is controlled by the chemist," Stoddart says.
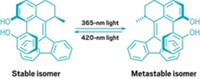
The motor consists of two components: a stationary aromatic base and a rotor component. The two pieces are connected by an alkene bond that acts as the motor's axle. Incident light causes a photochemical isomerization of the rotor, which begins to spin as it thermally relaxes to a more stable state. Continuous exposure to light causes repeated isomerization, which keeps the system rotating at about one revolution per second at elevated temperatures (around 80 °C), Feringa says.
When the pH of the system is increased, base-catalyzed epimerization alters the molecule such that incident light pushes the rotor in the opposite direction. The distinct feature of this reversible motor "is that by a simple stereochemical trick—namely base-mediated inversion at a single stereocenter—the directionality can be reversed without compromising the motor function," Feringa says.
Next up, the team is adjusting the structure to make the rotation faster. Ideally, the researchers would like it to complete one revolution every millisecond. Feringa says they are also planning to work on applications—for example, by introducing a variety of functional groups that control specific properties such as catalytic ability, both in a dynamic way and in response to light.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter