Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
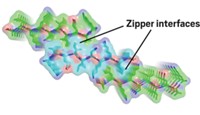
Water Factors In On Amyloid And Prion Aggregation Rates
Peptide aggregation in aqueous solution depends on the rate of formation of a dry interface between the biomolecules
by Stuart A. Borman
December 6, 2010
| A version of this story appeared in
Volume 88, Issue 49
When aggregation occurs in aqueous solution between amyloid or prion peptides—which are associated with protein-misfolding diseases—a dry interface between the biomolecules forms in two different ways, suggesting how aggregation rates might differ substantially (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1008616107). Theoretical models of aggregate formation created by Govardhan Reddy and Devarajan (Dave) Thirumalai of the University of Maryland and John E. Straub of Boston University show that when amyloid β peptide aggregates, fibril formation and expulsion of water from between the joining peptides occur virtually simultaneously. But when the more polar peptides from the yeast prion Sup35 aggregate, long-lived strings of water molecules called “water wires” form in the interface and have to be squeezed out for the dry interface to form, a process that takes additional time. Because of the different dewetting timescales of the amyloid and prion processes, “we surmise that amyloid fibrils can form nearly 1,000 times faster than prion fibrils,” the researchers write. The study elucidates “the role of water in amyloid fibril assembly and also points the way toward studying water in quasi-one-dimensional confinement,” comments theoretical biophysicist Gerhard Hummer of the National Institutes of Health.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter