Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Amantadine’s Double Bind
Mode of Action: New finding on flu drug’s binding to virus proton channel could aid drug design
by Stu Borman
February 8, 2010
| A version of this story appeared in
Volume 88, Issue 6

New details about how the flu drug amantadine works could ease the route to novel flu medications, of which there are currently painfully few.
Membrane protein specialist Mei Hong of Iowa State University and coworkers, including protein designer William F. DeGrado of the University of Pennsylvania, carried out the study (Nature 2010, 463, 689). They hoped it would resolve a controversy about the drug’s mechanism. But dissent persists.
The controversy goes back to independent studies in 2008 by Harvard Medical School structural biologist James J. Chou and DeGrado finding that amantadine and its sister drug rimantadine bind to different spots on influenza A’s M2 proton channel (Nature 2008, 451, 591 and 596). Because the drugs are so similar, the studies left uncertainty about both drugs’ site and mechanism of action.
The two drugs are approved but are no longer generally recommended because many flu strains have become resistant to them. Researchers would like to better understand M2-drug interactions to find a way around that problem. The only other approved flu drugs, oseltamivir and zanamivir, hit another viral target, neuraminidase.
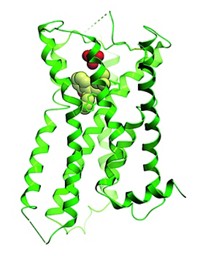
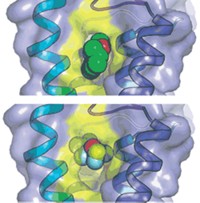
In the 2008 studies, DeGrado’s group obtained an X-ray structure of M2 that located amantadine in the channel, close to the residue that causes resistance, and they proposed that it worked by directly blocking the channel. Using solution NMR and an excess of rimantadine, Chou’s group found rimantadine outside the channel and proposed its mechanism to be allosteric.
Now, Hong and coworkers have used solid-state NMR to analyze the M2-amantadine interaction in phospholipid bilayers, which more closely resemble a cellular environment than do the micelles used previously. They find that at the pharmacologically optimal level of one molecule per channel, amantadine binds and rotates inside the channel, and that when excess amantadine is present, it binds both inside and outside the channel. “External binding occurs only with excess drug concentrations and is thus not pharmacologically relevant,” Hong says.
The findings “support previous functional and structural studies that show the pharmacologically relevant site to be in the channel’s pore, not at its cytoplasmic end,” comments neurobiology and physiology professor Lawrence Pinto of Northwestern University. “This knowledge should facilitate development of a useful drug.”
Molecular and cellular biology professor Robert A. Lamb, also of Northwestern, adds that the study “resolves the intellectual problem” about amantadine binding and that the allosteric proposal based on external binding “can now be put to sleep.”
But Chou disagrees. He believes it is unclear whether the newly observed channel interaction is an artifact of experimental conditions, and he doesn’t see how the drug can both rotate and bind specifically in the channel interior.
“The drug site that eventually leads to a new and better drug is the site that matters,” Chou says. “Let’s all wait and see which site that turns out to be.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter