Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Disease Enzyme Scrutinized
Structural Biology: X-ray structure of a conformationally flexible drug target reveals how its inhibitors work
by Sarah Everts
January 12, 2010

The first X-ray crystallography structure of a flexible member of an enzyme family involved in diseases ranging from cancer and diabetes to inflammation and asthma may bolster efforts to develop drugs that selectively target members of the family. Companies have long tried to develop drugs targeting the phosphoinositide-3-OH kinase (PI3K) family but have been stymied by the inability to target specific members and therefore minimize side effects from interactions with other members.
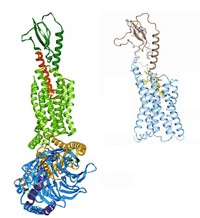
A team led by Roger L. Williams at the Medical Research Council's Laboratory for Molecular Biology in Cambridge, England, solved the structure of the δ isoform of PI3K bound to several inhibitors that were flat or shaped like propellers (Nat. Chem. Biol., DOI: 10.1038/nchembio.293). This isoform is often associated with inflammation. It has a topology that is similar to that of other PI3K isoforms whose structures have already been solved, but it appears to be more flexible than the others. The conformational flexibility may be the key to designing drugs that can specifically target this isoform, Williams says.
For example, the team found that the propeller-shaped inhibitors of the δ isoform squeeze into a site that is normally occupied by the energy-giving molecule ATP. In order to fit, the inhibitors create a new cavity in a section of the enzyme that is not normally open. "It's as if the enzyme swallows the propeller inhibitor, which then punches a hole in the roof of the enzyme's mouth," Williams says. If the δ isoform wasn't flexible, it would not be able to accommodate the inhibitor. Learning more about the conformational flexibility of enzymes that otherwise look the same may be key to designing drugs specific to a particular isoform, Williams adds.
The work is a "very important" contribution toward finding selective inhibitors, but "it is not the end of the story yet," notes Bernd Nürnberg, who studies PI3K enzymes at Eberhard Karls University, in Tübingen, Germany. "Companies have been working on these enzymes for more than a decade, and no compound has made it yet into Phase III clinical trials. These enzymes are more difficult than we expected," Nürnberg adds.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter