Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Surface Chemistry of Reverse Osmosis Membranes Slows Desalination
Wastewater Treatment: By tweaking charged functional groups on filter surfaces, researchers could improve efficiency
by Rajendrani Mukhopadhyay
October 4, 2010

In arid places such as Israel, Kuwait, and California, water treatment plants rely on reverse osmosis (RO) to desalinate waste or sea water for generating steam in thermal power plants and irrigating crops. A major hurdle in driving down operation costs and expanding desalination is removing mineral deposits from the membranes at the heart of the RO process. Now Israeli researchers have explored how the surface chemistry of these membranes trigger inorganic salt deposition and propose how to develop better membranes (Environ. Sci. Tech., DOI 10.1021/es101773t).
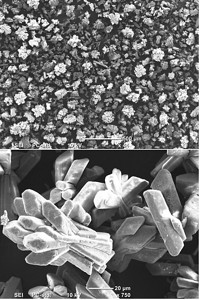
At desalination plants, pressurized water flows through RO membranes, which then filter out large molecules and ions. Municipal wastewaters can contain high concentrations of inorganic salts and silica that precipitate on to the membrane surfaces and form gel-like layers. This so-called scaling of RO filters increases desalination plants' operating costs because plant operators must increase the water pressure to overcome the deposit layer and achieve the same level of desalination.
Hydrochemist Roni Kasher, biotechnology engineer Hanna Rapaport, and colleagues at the Ben-Gurion University of the Negev in Israel wanted to understand how membrane surface chemistry triggers scaling. In particular, they focused on calcium phosphate because its relatively high concentrations in wastewater hinder recycling programs.
The researchers tested model organic surfaces decorated with functional groups similar to those on RO membranes, such as phosphate, hydroxyl, and amino groups. They exposed these model surfaces to a solution that mimicked the ion mixture found in wastewater effluents.
Kasher and his team then analyzed the deposits' compositions with Fourier transform infrared spectroscopy and X-ray diffraction. They found that calcium phosphate scaling rates were highest on surfaces with a net charge, such as those with excess phosphate or amino groups. But when the surface had equal numbers of positively- and negatively-charged groups, calcium phosphate scaling slowed significantly.
Kasher thinks that tweaking membrane surface group ratios could reduce scaling. Many of the RO membranes that he and his colleagues examined had a ratio of positively- to negatively-charged groups of 1:10. Manufactures could produce low-scaling membranes, he says, if they changed their preparations to produce a ratio closer to 1:1.
Environmental engineer Arup SenGupta of Lehigh University in Bethlehem, Pa., says scientists have long focused on the scaling problem and have proposed many solutions, but none have been technologically and economically feasible for large-scale use. But, SenGupta says, this study's proposed solution of bringing surface charge ratios closer to 1:1 "shouldn't be too difficult" to implement.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter