Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Rhodium-Catalyzed Reaction Pinned Down
Calculations suggest the C–H activation/Cope rearrangement combo can be customized to deliver multiple chiral products
by Carmen Drahl
March 14, 2011
| A version of this story appeared in
Volume 89, Issue 11
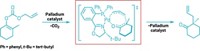
Through theoretical and experimental studies, chemists have learned how a catalytic asymmetric reaction called the combined C–H activation/Cope rearrangement works (J. Am. Chem. Soc., DOI: 10.1021/ja111408v). The reaction (shown) is generally selective for one stereochemical outcome, but the new work suggests it’s possible to obtain several products at will. The rhodium-mediated reaction is “a powerful transformation that has been used effectively in complex molecule assembly,” says organic chemist Joseph M. Fox of the University of Delaware. However, the mechanism for the process wasn’t clear before now. Emory University’s Jørn H. Hansen and Huw M. L. Davies, director of the NSF Center for Stereoselective C–H Functionalization, and colleagues determined that the transformation proceeds through a hydride transfer and carbon-carbon bond formation. Energy differences between the reaction’s possible transition states are small, which indicates that different chiral products should be obtainable in a controlled manner with modified reagents, something Davies’ lab has accomplished but not yet reported. This study “places the reaction on sound mechanistic footing and provides chemists with a predictive computational tool for understanding chemoselectivity and regioselectivity,” Fox says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter