Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Polymer Vision
ACS Meeting News: Chemists aim to create waterproof coatings that protect retinal implants without causing scarring
by Aaron A. Rowe
March 31, 2011
| A version of this story appeared in
Volume 89, Issue 14

By testing a range of materials, researchers at the University of Alabama, Huntsville, have come up with a set of waterproof coatings that both protect electronic retinal implants and the sensitive eye tissue of the wearer. The research was presented during a session in the Division of Polymer Chemistry on March 30 at the American Chemical Society national meeting in Anaheim.
Retinal implants could help millions of people with ocular disease regain some of their vision, but making a device that can send electrical signals from a camera to an array of electrodes in the eye is no easy task. If a bit of water gets into the electronics, they will short out.
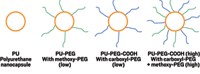
To solve this problem, Carmen Scholz and her colleagues are investigating a wide variety of protective coatings. The catch is that this impermeable material must be perfectly biocompatible.
"We don't want any scar formation," Scholz explained. Even a thin layer of damaged tissue could block the implant's faint electrical signals.
Initially, her team tested two polymers and two other substances by implanting them into pig eyes. Polyethylene glycol and diamond-like carbon performed better than amorphous aluminum oxide or polyvinyl pyrrolidone, which caused severe irritation, Scholz said. Since then, her group has focused on creating block copolymers made of polyethylene glycol and amino acid-based polymers. The researchers have also experimented with polyoxazoline as an alternative to polyethylene glycol.
"The work is wonderful," said Christopher Ober, who led the symposium session in which Scholz spoke. "It certainly shows the importance of polymer chemistry to interface between the material and the biological world."
Ober mentioned that a first-generation retinal implant has been tested in several people. He predicts that someday there will be smart coatings that can repair themselves, perhaps with some help from their surrounding tissue.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter