Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Picking Up The Pace Of Evolution
Molecular Biology: Method speeds lab-based molecular evolution
by Celia Henry Arnaud
April 18, 2011
| A version of this story appeared in
Volume 89, Issue 16
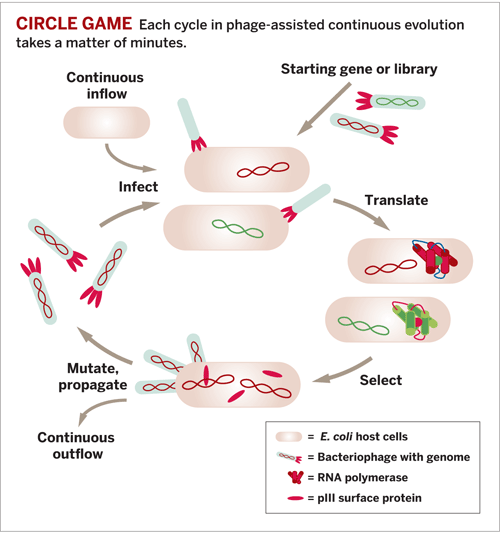
A new system for directed evolution of biomolecules, a technique that leads to compounds with improved properties, works in a fraction of the time of previous methods, Harvard University chemists report (Nature, DOI: 10.1038/nature09929). The method could lead to biomolecules for new therapeutics and to a better understanding of natural evolution.
The technique, phage-assisted continuous evolution (PACE), uses bacteriophages (viruses that infect bacteria) to express the desired biological molecules. It was developed by chemistry professor David R. Liu and grad students Kevin M. Esvelt and Jacob C. Carlson.
Phages are well suited for directed evolution of biomolecules because the phage life cycle proceeds extremely rapidly and can be linked to a desired activity of the biomolecule.
In PACE, Escherichia coli cells flow through a “lagoon”—a fixed-volume vessel containing phages that can replicate only if they infect the bacteria and carry genes encoding molecules with desired activities.
The gene for an evolving biomolecule, which is added to the phage genome, changes in each replication cycle. And the gene for a phage surface protein called pIII, which the phage needs to infect bacteria, is removed from the phage and added to an “accessory plasmid” in E. coli.
The bacteria express pIII at a rate proportional to the activity of the evolving biomolecule. So phages that encode biomolecules with more of the desired activity cause the bacteria they infect to express more pIII, resulting in the production of progeny phage that can go on to infect other host cells and persist in the lagoon. Less active biomolecules result in less infectious phage that cannot propagate fast enough to survive the constant outflow from the lagoon.
In the first examples of PACE, the researchers evolved RNA polymerase to adopt desired new properties. In principle, any biomolecule with properties that can be linked to pIII production can be evolved in this way, Liu says.
The phage population can go through approximately 30 rounds of evolution in a single day and more than 200 rounds in a week. With conventional methods of directed evolution, a single round can take several days.
“This is the first laboratory system for the controlled, continuous evolution of proteins,” says Gerald F. Joyce, a chemist studying directed evolution at Scripps Research Institute.
When Liu and coworkers conducted PACE on the same biomolecule evolving simultaneously in two lagoons under identical conditions, key mutations evolved quickly in one lagoon but not the other. Still, results were similar. This shows that the method can be used to study details about the way molecular evolution occurs, the researchers say.
“We’re running many lagoons in parallel, asking, ‘If you replay long evolutionary trajectories—the “tape of life,” as Stephen Jay Gould called it—over and over again under different conditions, what happens?’ ” Liu says.
The system is “quite ingenious,” says Donald Hilvert, who studies directed evolution at the Swiss Federal Institute of Technology, Zurich (ETH). “If it is as robust as it seems, it could become a broadly useful tool for evolution in the lab.” However, Hilvert notes, it’s not clear yet “how finicky the selection protocol is or how easily artifacts arise.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter