Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Mending With Light
Materials Chemistry: Light prompts metallosupramolecular polymers to repair themselves
by Bethany Halford
April 25, 2011
| A version of this story appeared in
Volume 89, Issue 17

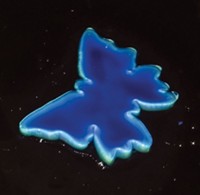
Scratches, cuts, and cracks, although they may seem small, can add up to big bucks when they’re in polymer coatings. Just ask anyone who’s found a scratch to their car’s paint job. Thanks to a family of metallosupramolecular polymers, spot-repairing damage to paints, coatings, and polymer thin films could one day be as simple as shining an ultraviolet light on them (Nature, DOI: 10.1038/nature09963).
The self-healing material, which comes from a group led by Stuart J. Rowan of Case Western Reserve University and Christoph Weder of the University of Fribourg, in Switzerland, is based on short polymer chains that terminate in a ligand that can coordinate to a metal ion. “When we put in a metal ion—in this case either zinc or lanthanum—the components bind to the metal and essentially form a high-molecular-weight polymer,” Rowan explains.
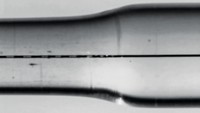
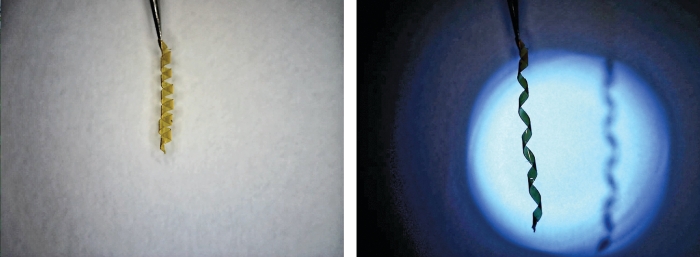
When light shines on the polymer, the metal-binding ligands absorb the light’s energy and convert it into heat, which, in turn, makes the metal ion break ties with the ligands. “What you’re doing is depolymerizing the system using this photothermal process,” Rowan says. The depolymerized material is liquid and can flow into and heal cracks or scratches. “When you remove the light, the ligands rebind to the metal, polymerizing again and reestablishing the mechanical strength of the material,” he adds. All of which takes place in under a minute.
There are other light-activated self-healing materials, but they rely on embedded particles or agents, which can be exhausted, or they use irreversible chemical reactions for healing. “We’ve used a reversible reaction,” Rowan says, “so you can scratch and heal and scratch and heal.”
The work “represents a major step forward in the nascent field of self-healing polymers,” comments Michael R. Kessler, a materials science and engineering professor at Iowa State University. “What makes this approach potentially so useful is that the light can be directed locally at the damaged region, so that bonds are only re-formed where the damage occurs, allowing the undamaged material to continue to carry load during the healing process.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter