Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Multimetal Organic Complexes
Synthesis: Method couples various metals in predetermined sequences
by Mitch Jacoby
January 10, 2011
| A version of this story appeared in
Volume 89, Issue 2
Making molecules that contain multiple metal atoms and more than one type of metal is notoriously tough. But that’s exactly what a research team based in Japan and the U.S. has done.
The researchers have devised a method to string metal-containing molecular segments in specific sequences and lengths to form surface-tethered metal-organic complexes, including one with three types of metals and a total of six metal atoms (J. Am. Chem. Soc., DOI: 10.1021/ja1097644). Complexes of this type may mediate highly selective catalytic reactions or “cascading reactions.”
The underlying design strategy is an adaptation of the Merrifield solid-phase peptide synthesis, according to Omar M. Yaghi of UCLA, who led the study.
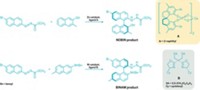
To make the complexes, Yaghi; Kentaro Tashiro of the National Institute for Materials Science, in Tsukuba, Japan; and coworkers functionalized tyrosine with a multidentate ligand and reacted the product with platinum, rhodium, or ruthenium. Then they attached one of those building blocks to a polymeric resin and linked additional building blocks to the surface-bound unit to tailor-make products featuring a desired number of metal atoms in a particular sequence. For example, they prepared one of the complexes by coupling units containing Rh, Pt, Ru, Pt, Rh, Pt—in that order.
“The idea of extending the Merrifield solid-phase synthesis to metal-coordination complexes to create predetermined and varying metal sequences is brilliant,” says Northwestern University’s Mercouri G. Kanatzidis, a materials chemistry specialist.
This technique has the potential to inspire broad-based activity in tailor-made complexes for specific applications, Kanatzidis says. “I am curious to see how people will use this approach. Who knows,” he laughs, “finally we may be able to synthesize the Co-Ca-Co-La molecule.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter