Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Ubiquitins In Four-Part Harmony
Biomacromolecular Synthesis: Tetraubiquitin is the longest protein made chemically
by Stu Borman
May 30, 2011
| A version of this story appeared in
Volume 89, Issue 22

By chemically preparing a four-membered chain of ubiquitin proteins for the first time, a research team has not only opened the door to a better understanding of the role of polyubiquitins in biology but also set a new size record for chemical synthesis of proteins.
Different types of polyubiquitins have different cellular functions, such as ferrying unneeded proteins to the proteasome, the cell’s recycling center. There are seven homogeneous polyubiquitins, consisting of individual units linked to one another via the same lysine residue. But there are also innumerable possibilities for mixed-linkage types of polyubiquitins.
Scientists would love to better understand the relationship between polyubiquitin structure and function. But this has been difficult because polyubiquitins are hard to synthesize. Enzymatic techniques have yielded only a few types of homogeneous tetraubiquitins.
Tetraubiquitins are of particular interest because they are believed to be the smallest polyubiquitins that are active biologically. For instance, homogeneous tetraubiquitin attaches to proteins to mark them for disposal by the proteasome. But polyubiquitins this large have not previously been made by chemical synthesis, which has the potential to produce versions that are much more customized than those accessible enzymatically.
Chemical synthesis has until now been restricted to diubiquitins, which several groups created last year (C&EN, Oct. 11, 2010, page 36). One group, led by protein synthesis specialist Ashraf Brik of Ben-Gurion University of the Negev, in Israel, prepared all seven types of homogeneous lysine-linked diubiquitin chains (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201003763).
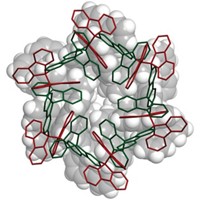
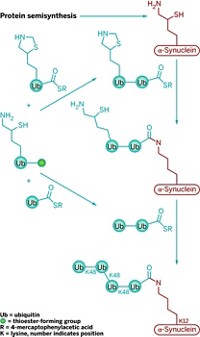
Now, Brik and coworkers report having extended that work by using a similar chemical approach to synthesize a homogeneous tetraubiquitin (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201101920). Prior to this feat, the largest protein molecules prepared by total chemical synthesis had about 200 residues. Tetraubiquitin has 306, making the new structure the largest protein made chemically.
The researchers believe the technique can be further extended to other types of homogeneous and mixed-linkage tetraubiquitins and perhaps longer polyubiquitins as well. “By using chemical synthesis, virtually unlimited variations” would be accessible, the researchers write in their latest paper. “This would lead to unraveling of the thus-far unattainable details of ubiquitin biology.”
“The limited availability of polyubiquitin chains of defined lengths and linkage composition certainly has been a major impediment to the ubiquitin field,” comments polyubiquitin expert Robert E. Cohen of Colorado State University. “The total chemical synthesis approach that’s now been developed may provide the ultimate solution.”
To make tetraubiquitin, Brik and coworkers first tried a convergent approach—synthesizing two diubiquitins and combining them—which they thought might be more efficient than linear synthesis. But they ran into problems and ended up using linear synthesis to connect four ubiquitin units with native isopeptide bonds at 5% isolated yield. They then showed that the synthesized tetraubiquitin folds into its native protein form.
In principle, the approach “offers complete flexibility with respect to ubiquitin-ubiquitin linkage type and has the potential for incorporation of nonnatural amino acids and other modifications,” Cohen notes. “It could have an enormous impact on the field.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter