Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Promising Pom-Pom
Therapeutics: Nucleic acid structures enter cells readily, control gene expression
by Stu Borman
June 20, 2011
| A version of this story appeared in
Volume 89, Issue 25

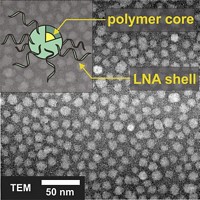
A research team at Northwestern University reports a new form of synthetic DNA and RNA that looks like cheerleader pom-poms and can enter cells readily and regulate genes (J. Am. Chem. Soc., DOI: 10.1021/ja203375n). These polyvalent nucleic acid nanostructures (PNANs) could prove to have therapeutic effects by controlling the expression of disease-related genes, their creators say.
Nanoscience expert Chad A. Mirkin and coworkers at Northwestern University developed PNANs and are collaboratively pursuing their use to treat a wide variety of diseases with genetic bases, including glioblastoma, a form of brain tumor for which new therapies are needed. They also are investigating the use of PNANs to speed wound healing and treat psoriasis, neurological diseases, and cardiovascular conditions, Mirkin says.
PNANs are composed of DNA and/or RNA strands that radiate from a central point, where they are synthetically cross-linked. Synthetic DNA and RNA oligomers and PNANs can inhibit cellular gene expression by blocking mRNA-to-protein translation. But synthetic DNA and RNA strands typically do not enter cells readily, Mirkin says. That’s because single strands don’t interact efficiently with cell-surface receptors that control endocytosis, the process by which cells engulf extracellular molecules, he explains. PNANs enter cells more easily because their high nucleic acid surface densities, oriented strands, and large particle sizes allow them to interact efficiently with the endocytosis-inducing gatekeepers on cell surfaces.
Transfection agents such as polymers, liposomes, peptides, or viruses can enhance entry of synthetic DNAs and RNAs into cells. But these carriers can be toxic, immunogenic, and resistant to breakdown and may therefore cause side effects. By obviating the need for transfection agents, PNANs take concerns about transfection agent toxicity and side effects “completely out of the equation,” comments Dan Feldheim of the University of Colorado, Boulder, a specialist in medicinal nanostructures.
Another important advantage of PNANs is that they resist enzymatic breakdown by nucleases. This enables them to last longer in the body—and more effectively knock down genes—than most synthetic DNA and RNA strands.
In their paper, Mirkin and coworkers demonstrate that RNA-based PNANs can enter squamous cancer cells and inhibit the expression of epidermal growth factor receptor via an RNA interference mechanism.
PNANs are actually core-free versions of constructs Mirkin’s group developed earlier—DNA and RNA oligomers attached to gold-nanoparticle cores, which also enter cells readily.
“I was excited to see that the gold core could be removed so easily without compromising the function of the oligonucleotide shell,” Feldheim says.
Nanomedicine specialist Joseph M. DeSimone of the University of North Carolina, Chapel Hill, says PNANs could “change the way people think” about nucleic acid therapeutics. They could “open up a lot of new approaches,” he says. “People are clearly going to try to build on this and see what other nucleic acid complexes one can make that will undergo similar cell internalization.”—Stu Borman


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter