Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Structure Of Brassinolide’s Receptor Solved
Agriculture: First look at sensor for plant hormone holds surprises
by Sarah Everts
June 20, 2011
| A version of this story appeared in
Volume 89, Issue 25

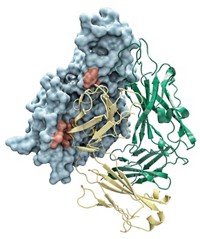
The first X-ray crystal structures of a key plant hormone receptor protein have been solved by two independent research teams working in the U.S. and China (Nature, DOI: 10.1038/nature10178 and 10.1038/nature10153). The receptor senses brassinolide, a steroid that helps bulk up foliage, fight pathogens, and mediate fertilization. It is among the last of the major plant hormone receptors to have its structure solved.
The discovery sets the stage for plant researchers to improve the yields of lettuce and cotton, two crops that depend on brassinolide signaling for large harvests. The structure will also help plant scientists understand and bolster agricultural crops’ immunity to microbial pathogens.
Surprisingly, the brassinolide receptor “looks totally different than what was expected,” says Ping He, a plant biochemist at Texas A&M University. People so strongly believed the receptor would adopt a horseshoe shape, He explains, that the wrong structure was widely “put in textbooks.”
Instead, the brassinolide receptor, called BRI1, adopts a superhelix conformation that sequesters the hormone inside the ring, with the help of an “island” domain, so named because it appears to float in the center of the receptor’s circular interior.
The two research teams—one led by structural biologist Jijie Chai at Tsinghua University, in Beijing, and the other by Joanne Chory, a plant biologist at the Salk Institute, in La Jolla, Calif.—knew of each other’s work, but did not share data prior to submitting the papers for publication. When Chai finally read Chory’s paper, “I could not find a difference between their structure and ours,” he says. The impressive similarity “certainly helped” convince reviewers who were expecting BRI1 to look like its mammalian analog, the horseshoe-shaped steroid receptor called TLR3, Chory says.
The researchers were also surprised to find that BRI1 does not seem to dimerize as TLR3 does, Chai says.
Chemists could use BRI1’s structure to develop small, receptor-activating molecules that aren’t as complicated—and therefore not as expensive to produce or purify—as brassinolide. The structure might also help plant scientists engineer the receptor to increase crop sensitivity to brassinolide, thus speeding the launch of defensive strategies against invading microbial pests, He adds




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter