Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Thermoelectrics Make A Comeback
New concepts and materials invigorate a commercially active but obscure field specializing in heating, cooling, and power generation
by Mitch Jacoby
June 20, 2011
| A version of this story appeared in
Volume 89, Issue 25

“Ten or 15 years ago, nobody wanted to hear about thermoelectrics. Back then, people couldn’t even spell the word.”
With playful exaggeration, Mercouri G. Kanatzidis, a Northwestern University chemistry professor and materials specialist, makes the point that during the past decade, the field of thermoelectrics—which encompasses a collection of heating, cooling, and power generation technologies enabled by a unique class of semiconductors—is undergoing a renaissance.
In the early 1960s, long after the curious collection of properties that define thermoelectric materials was discovered, manufacturers began producing specialty cooling and power-supply devices largely based on the thermoelectric properties of bismuth telluride, Bi2Te3. This niche market, which mainly served the military and aerospace industry, didn’t disappear, but the next 30-plus years witnessed a steep drop in interest in the topic.
“In the mid-1990s, universities rarely taught thermoelectrics in physics courses,” Kanatzidis points out. He adds, “It was a forgotten concept. Now it’s finally coming of age.”
After years of sitting mostly unnoticed in a quiet corner of science, thermoelectrics is again drawing attention. In addition to supplying temperature management and power products to the military and aerospace industry—for example as miniature coolers that chill the infrared detectors central to the imaging electronics in heat-seeking missiles and night-vision systems—the few manufacturers in this area now make millions of units each year for down-to-earth civilian use. Their products are found in climate-control automobile seats offered by major automakers, thermal cyclers for polymerase chain reaction systems, and power generators for applications far from an electrical grid.
At the same time, researchers in industry and academia, motivated by recent fundamental materials advances, are focusing their synthesis, analytical, and engineering skills on discovering new thermoelectric materials and designing new ways to use them. They hope these advances will provide enhanced thermoelectric performance and lead to a broader range of products. Despite key advantages of thermoelectric power and cooling systems relative to conventional ones—for example, they have no moving parts, making them mechanically simpler, and they do not emit greenhouse gases or depend on environmentally harmful coolant fluids—only a narrow range of thermoelectric products has been commercialized. One of the field’s much talked about goals is to make thermoelectric devices that work well at high temperature and can generate electricity cost-effectively from waste heat recovered, for example, from industrial plants or automobile exhaust.
The key observations that underpin the thermoelectric effect were made way back in the early 1800s. In 1821, German physicist Thomas Johann Seebeck discovered that if a loop made from dissimilar metals is exposed to a temperature gradient (one side of the loop warm, the other cool) the loop can deflect a nearby compass needle. That set of conditions generates an electrical current and a magnetic field.
Another key observation was made by French physicist Jean-Charles Peltier in 1834. Peltier found that if a current is applied across a junction of dissimilar electrically conductive materials, the junction can heat up or cool down. Reversing the current flow, by switching the battery hookups, for example, results in the opposite heating or cooling effect.
In most materials, the properties that cause a temperature change as a result of an applied current are too weak to be useful for thermoelectric applications. The same is true for those that generate an electrical current in response to a temperature gradient.
In fact, materials that exhibit a pronounced thermoelectric effect are rather exotic, according to Lon E. Bell. Bell founded thermoelectrics manufacturer Amerigon, based in Northville, Mich., and later the wholly owned thermoelectrics subsidiary, BSST, in Irwindale, Calif. For materials to be useful thermoelectrically, he explains, they need to be good electrical conductors and, at the same time, poor thermal conductors. They also must prominently display the effect observed by Seebeck.
One of the challenges in finding useful materials is that the desired qualities are interrelated via the substance’s electronic properties: Improvement in one property often comes at the expense of another. In these materials, electrons serve as the “working fluid,” just as liquid refrigerants do in conventional air-conditioning systems. In a thermoelectric cooling application, for example, the IR detector, circuit component, or other item that needs to be cooled sits in contact with one face of the cooling device. Electrons at that face shuttle heat away from the item that’s being cooled through the thermoelectric material and deposit it at the thermoelectric material’s other face. There, the heat dissipates—sometimes with the help of a fan.
Kanatzidis likens the process to electrons carrying bundles of heat down a corridor. When the corridor is mostly empty, each electron can deliver its load easily, which is good for cooling applications. But if the corridor has a few electrons milling about, the material’s electrical conductivity will be low. Raising the electrical conductivity means getting more electrons moving through the corridor. But as Kanatzidis explains, all that activity leads to congestion and causes electrons to bump into each other, scatter, and drop their bundles of heat. As a result, the material equilibrates ther mally.

The tricky thing is finding materials that can maintain a large temperature difference across the two sides of a slab of the material. If the thermal conductivity is too high, the temperature difference goes away and the thermoelectric effect becomes too small to be useful. “Optimizing the parameters in a thermoelectric material is a matter of compromise,” Kanatzidis says. “You need to find a happy medium.”
Bismuth telluride is one of the first materials found to strike the necessary compromise. Its so-called thermoelectric figure of merit—ZT, a dimensionless quantity that indicates how intensely a material exhibits the thermoelectric effect—is about 1.0 at room temperature. To put the material to use practically, for example in a power generator, small chunks of the material that have been doped to render them positive- and negative-charge-carrying semiconductors (p-type and n-type, respectively) are joined electrically to form a circuit. Large numbers of these p-type and n-type pairs are connected in series, forming thermoelectric arrays that can provide large voltages and mediate substantial heat flow.
Although decades ago proponents were able to point to a select number of impressive accomplishments in the field of thermoelectrics, such as reliable power generators for U.S. and Soviet deep-space vehicles, few advances were made between 1960 and the early 1990s. As Mildred S. Dresselhaus recounts the story, one of the key events that helped reawaken the field grew out of an early-1990s dinner conversation at a restaurant in Belgium.
Dresselhaus, a professor of physics and electrical engineering at Massachusetts Institute of Technology, recalls how she chatted over dinner that evening with Jean-Paul Issi of Belgium’s Catholic University of Louvain and others, including a senior representative of the French Navy, about strategies to improve the performance of thermoelectric materials. At that time, the U.S. and French Navies were both looking into new ways to power submarines, and the dinner conversation soon turned to thermoelectrics. Dresselhaus hit upon the idea that nanoscale materials might have promising thermoelectric properties and decided to look into the topic.
A year or so later, she published a paper with graduate student Lyndon D. Hicks in which they showed theoretically that decreasing the thickness of a three-dimensional material—that is, making thinner and thinner films—increases the material’s ZT (Phys. Rev. B, DOI: 10.1103/PhysRevB.47.12727).
SLOPPY CRYSTALS
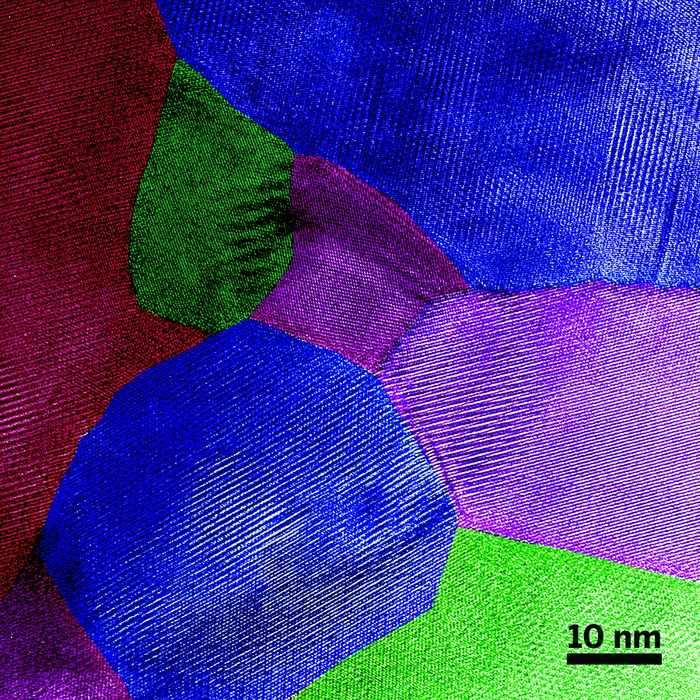
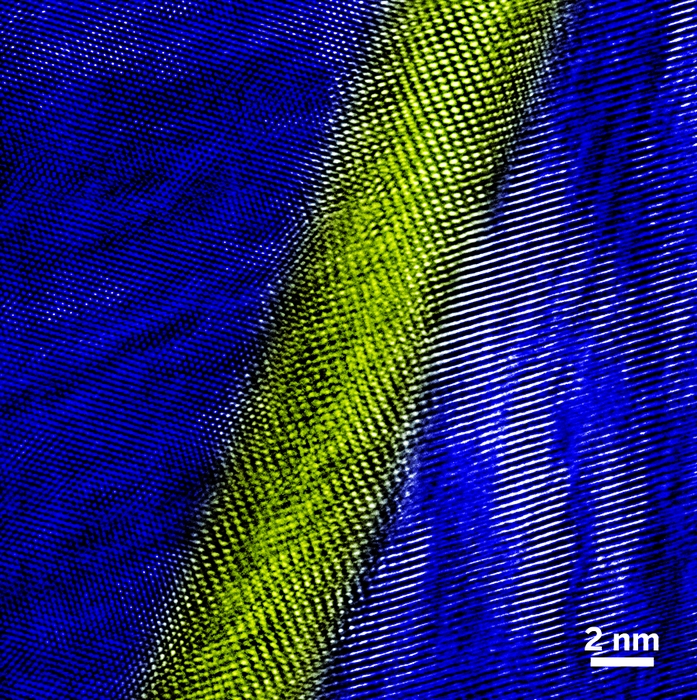
The random orientation of microscopic grains (colored patches top) in this bismuth antimony telluride specimen and nanometer-wide bismuth-rich regions in between the grains (yellow area below) play a key role in this material’s thermoelectric properties.
“Since the idea worked so well in 2-D, we decided to do the same calculation in 1-D,” meaning on a nanowire, Dresselhaus says. The pair found that the additional confinement imposed by a nanowire relative to a thin film led to even better results and again published their findings in Physical Review B (DOI: 10.1103/PhysRevB.47.16631). Progress was slow because few people were working in the field at that time, Dresselhaus says. She adds that eventually others picked up on the nanoscale-confinement idea and produced experimental results supporting the theoretical predictions.
Meanwhile, Kanatzidis continued working to discover new bulk thermoelectric materials. His aim was to use solid-state synthesis methods to customize the composition of promising materials and thereby tune their thermoelectric properties. Eventually, that approach paid off. In 2004, Kanatzidis’ group found that samples of lead telluride containing antimony and silver (AgPbmSbTe2+m) are characterized by remarkably high figures of merit—up to 2.2 at around 500 °C, which is nearly twice the value of previous record holders (Science, DOI: 10.1126/science.1092963).
Although the findings were exciting, Kanatzidis and coworkers were initially at a loss to explain them. The breakthrough came from transmission electron microscopy (TEM) results indicating that the high-performance materials were rather inhomogeneous and full of nanometer-sized crystallites or precipitates rich in silver and antimony embedded in a lead telluride-rich matrix. Because of their structures and electronic properties, the precipitates block the propagation of heat-carrying lattice vibrations known as phonons, thereby reducing the material’s thermal conductivity, Kanatzidis explains.
Building on those results, Kanatzidis’ group devised synthesis methods that cause numerous nanocrystals of SrTe to precipitate inside a PbTe matrix such that both sets of lattices match perfectly in 3-D. As reported in a paper published earlier this year, the structure and composition of these “perfect” synthetic lattices block the propagation of phonons, but they do not impede the transport of charge typically caused by embedded crystallites. This “rational design” approach led to a ZT value of 1.7 near 500 °C (Nat. Chem., DOI: 10.1038/nchem.955).
Investigations by Kanatzidis, Dresselhaus, and others have stimulated a recent rise in thermoelectrics research that is helping uncover new structural and electronic phenomena as well as novel types of promising materials. For example, in a TEM study of bismuth antimony telluride samples with a ZT of 1.4, Yucheng Lan and Zhifeng Ren of Boston College, working with MIT’s Gang Chen, found that the bulk material contains numerous randomly oriented nanosized grains dotted with precipitates. They also detected bismuth-rich regions several nanometers thick in between the grains (Nano Lett., DOI: 10.1021/nl803235n).
And just recently, a group led by California Institute of Technology materials science professor G. Jeffrey Snyder showed that ZT values as high as 1.8 could be coaxed from lead telluride (often characterized by ZT below 1.0) by selectively doping the material with sodium and selenium in a way that customizes the material’s electronic structure. Specifically, the group reports that samples of Pb0.98Na0.02Te1–xSex benefit from a high “valley degeneracy.” In terms of the electrons-in-the-corridor analogy, this material can be thought of as having multiple parallel corridors, which helps avoid electronic congestion (C&EN, May 9, page 38).
In addition to the commonly studied materials—ones based on bismuth telluride, lead telluride, and related compounds—other classes of materials also figure into today’s research on thermoelectrics. At the University of California, Berkeley, for example, chemistry professor Peidong Yang has shown that In2- xGaxO3(ZnO)n nanowires and holey silicon membranes, both of which can be prepared via straightforward synthesis methods, show promise as thermoelectric materials.
Yet another group of materials, skutterudites, which are cobalt-antimony-based compounds containing rare-earth elements, figure prominently into research aimed at generating electricity from waste heat. Skutterudites appear to be well suited for this application because, unlike bismuth telluride, they have high ZTs at the high temperatures typical of industrial and automobile exhaust systems. As part of a multiyear project partly sponsored by the Department of Energy, Gregory P. Meisner, James R. Salvador, and coworkers at General Motors have been developing prototype devices that use automobile exhaust heat to produce electricity for onboard use.
Advertisement
The team’s work, carried out with various partners including Dallas-based Marlow Industries, shows that such devices can readily provide fuel-economy improvements of several percent. With further development of hybrid cars and other types of electric vehicles, even greater benefits can be realized from such a system, Meisner says.
“Discovery of new materials has really propelled this field forward,” Meisner asserts. He adds that progress is now being made quickly across all of thermoelectrics. Yet he injects a note of caution by pointing out that key engineering problems still need to be solved. In the case of exhaust-heat recovery, for example, the thermoelectric system must be highly robust and heat resistant. Furthermore, its weight, volume, and cost all must be optimized, he adds.
As Amerigon and BSST’s founder, Bell, sees it, if those types of limitations can be overcome, “thermoelectrics could well play a crucial role in addressing some of the sustainability issues we face today.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter