Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cocrystal Engineering Sorts Out Ladderanes
by Stephen K. Ritter
July 18, 2011
| A version of this story appeared in
Volume 89, Issue 29
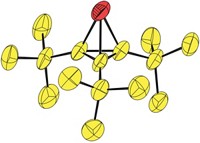
A cocrystallization strategy commonly used in protein chemistry has been redirected to help elucidate the structures of a pair of complex ladderane isomers, possibly the first application of the technique to the structural characterization of small organic molecules (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1104352108). Ladderanes are rod-shaped molecules that contain a core set of fused cyclobutane rings. They are used as building blocks in optoelectronics and recently were discovered to be a structural motif in lipid natural products. Leonard R. MacGillivray and coworkers of the University of Iowa had prepared a pyridyl-terminated ladderane, but they were unable to pin down the structures of the molecule’s cis-trans isomers by the standard approach of multidimensional NMR. Although the researchers managed to obtain the X-ray crystal structure of one isomer, which is achiral, the other isomer, which is chiral, refused to cooperate, forming only an amorphous material. But adding 3,5-dinitrobenzoic acid, which forms hydrogen bonds with the ladderane’s pyridyl groups (shown), led to single crystals for the X-ray analysis. “We expect our work will spur others to follow similar cocrystallization strategies in synthetic organic chemistry,” MacGillivray says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter