Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
High-Throughput Reaction Discovery
Organic Chemistry: Method lets chemists study thousands of transformations simultaneously
by Bethany Halford
September 12, 2011
| A version of this story appeared in
Volume 89, Issue 37

Thanks to a new approach, chemists can now run thousands of experiments with the hope of finding new coupling transformations, all in just a matter of days. The high-throughput method allows researchers to vary catalysts, ligands, and multiple substrates all at one time using simple laboratory equipment and mass spectrometry analysis (Science, DOI: 10.1126/sci ence.1207922).
“It’s very simple,” says University of California, Berkeley, chemistry professor John F. Hartwig, who developed the approach with graduate student Daniel F. Robbins while the two were at the University of Illinois, Urbana-Champaign. “We hope that it’s a method that can be used by labs besides our own.”
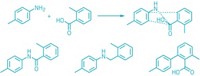
To screen for new coupling reactions, the chemists took four 96-well plates and loaded each of the wells with a transition-metal-catalyst precursor, a ligand, and 17 substrates, each carrying one functional group. “We chose substrates that have about the same molecular weight so if they couple we can distinguish the products from the reactants by mass spectrometry,” Hartwig explains.
To eliminate the need to run an MS analysis for each well, Hartwig and Robbins developed a method to analyze representative samples from each row and each column of a plate. They then use their MS data to pinpoint wells with products.
In cases where they cannot directly deduce from the MS data which of the 17 substrates reacted, they split the substrates into four groups and rerun the reaction. They repeat this winnowing process until the reacting substrates are identified. Hartwig says he got the idea from trying to identify which portion of a Microsoft Word document was making his computer crash. Rather than go through the document page by page, he repeatedly split it in half, identifying the bad half and ignoring the rest.
“The method is clever and effective without being sophisticated, and can be easily adopted by others without special equipment or expertise,” writes University of Michigan chemistry professor John Montgomery in a commentary that accompanied the piece.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter