Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
The Bryostatins’ Tale
With the promise of treating cancer, Alzheimer’s, and HIV this family of marine natural products continues to intrigue scientists more than four decades after its discovery
by Bethany Halford
October 24, 2011
| A version of this story appeared in
Volume 89, Issue 43

One June day in 1968 marine biologist Jack Rudloe went down to collect specimens from the docks at his local marina on the northern Florida coast of the Gulf of Mexico. As the man behind Gulf Specimen Co., Rudloe was used to catching some of the Gulf’s more interesting creatures—electric rays, bonnethead sharks, and live jellyfish—for aquariums and research centers throughout the country. But on this day, his mission was simpler: Gather some marine organisms that were abundant and easy to collect (inexpensive, in other words) and send them to Jonathan L. Hartwell’s anticancer drug discovery group at the National Cancer Institute(NCI).
Among the dozen organisms Rudloe collected “shotgun style” that day, was a small brownish spray that looked like seaweed. Despite its appearance, the material was not a plant but rather a colony of the bryozoan Bugula neritina, tiny filter-feeding critters, each about a millimeter long that clump together in a branching structure. B. neritina is, in fact, a pest that fouls floating docks and boats in waters worldwide.
Rudloe put a few handfuls of the bryozoan through a meat grinder, packed it in isopropyl alcohol, and sent it to Frederick, Md. “It was just sheer luck,” he says, that he had picked an organism armed with molecules that could fight cancer, Alzheimer’s disease, and HIV.
Those compounds are the bryostatins, a family of 20 macrolide lactones, 18 of which have been structurally characterized. Since they were first plucked from obscurity more than 40 years ago, the compounds have had a colorful history. They were hailed as key compounds in the fight against cancer, but over the years, bryostatin 1, the most-studied member of the family, failed to impress. In more than three dozen clinical trials to fight various forms of the disease, it gave mostly mediocre results, both on its own and in combinations with other cancer-fighting drugs.
The compounds have also fallen out of favor as drug candidates for a more practical reason: Harvesting them from the naturally occurring bryozoan is impractical, and their long chemical syntheses were too unwieldy for drugmakers.
Recently, however, the cloud that was hanging over the bryostatins has begun to lift. Animal tests show that bryostatin 1 enhances memory and could be used to treat Alzheimer’s disease and strokes. And some preliminary studies show it could help eradicate HIV. What’s more, chemists have dramatically whittled down the number of steps it takes to make these molecules. This year, three total syntheses of bryostatin natural products were published, with the shortest being just 36 steps. Finally, as chemists have found a way make bryostatins faster, there’s been a push to make analogs of these compounds so that scientists might get a better handle on how they operate biologically and make simpler molecules that would be more practical drug candidates.
George R. (Bob) Pettit, a natural products expert and chemistry professor at Arizona State University, was one of the people driving research to find cancer-fighting agents from marine organisms back when Rudloe scooped those first handfuls of B. neritina. In the early 1970s Pettit began collecting bryozoans from the Gulf of California, in Mexico, and the Sagami Gulf, in Japan. But it was extracts from a sample of B. neritina taken from the California coast that most interested Pettit and the folks at NCI.
Pettit’s group spent most of the late 1970s trying to isolate the compounds responsible for the antineoplastic activity in bryozoan extracts. By 1981, Cherry L. Herald, a scientist working in Pettit’s lab, had isolated the first milligram of what would be known as bryostatin 1 from that California collection of B. neritina. “I dashed it off to the National Cancer Institute and the activity was tremendous,” Pettit recalls. “It was clear we had to determine the structure.”
Using 500 kg of B. neritina, Pettit’s research team isolated 120 mg of bryostatin 1. They crystallized the material and determined the compound’s structure, which they reported in 1982 (J. Am. Chem. Soc., DOI: 10.1021/ja00388a092). “We were blessed,” Pettit says of the ease with which they crystallized the compound.
The structures of 17 other bryostatins would follow (extracts from Rudloe’s samples became known as bryostatins 4 through 8). But it was bryostatin 1 that NCI began to focus on as a potential drug. In 1991 the institute undertook a massive isolation of bryostatin 1 from B. neritina off the California coast, collecting some 14 tons of the animal, which Pettit recalls shipping to NCI in 120 55-gal drums.
From those 14 tons, researchers isolated 18 g of bryostatin 1 (J. Nat. Prod., DOI: 10.1021/np50077a004). That’s enough to fill a typical salt shaker up just a quarter of an inch, Pettit estimates. Nevertheless, because bryostatin 1 is so potent, those 18 g have been enough to supply all the clinical trials using the compound.
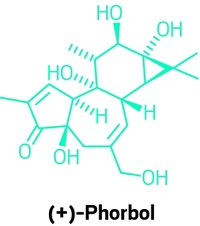
Bryostatin 1 works by modulating the activities of a family of enzymes known as protein kinase C, or PKCs. Once activated, these enzymes phosphorylate certain proteins and play an important role in intracellular signaling cascades. PKCs first attracted the attention of biologists because they are the target of phorbol esters, the archetypal tumor promoters.
But while phorbol esters make tumors grow like crazy, bryostatin 1 suppresses tumor growth—even though they both bind to the same part of PKCs. It’s a phenomenon that still puzzles biologists. “Of the known activators of PKC, bryostatin 1 is the only known agent that is a functional antagonist of most phorbol ester functions,” says Gary E. Keck, a chemistry professor at the University of Utah who has been studying the bryostatins for the past decade.
In fact, a number of natural products activate PKCs. Like bryostatin 1, they bind to a region of the enzymes known as the C1 domain. When a small molecule fills this C1 cleft, a PKC enzyme opens to receive its substrates. The binding also makes the C1 region hydrophobic, enabling PKC to move from the cytosol, where it resides in the absence of activation, to a membrane. That membrane could be the cell membrane, the nuclear membrane, or membranes of other cell organelles. Once stuck to the membrane, PKC finds its protein targets and phosphorylates them, setting off the signaling cascade.
PKC-activated proteins are involved in some of the most important cellular functions, Keck points out. They make cells grow. They make cells morph into different kinds of cells. And they are involved in apoptosis, or programmed cell death. “The most critical processes of the cell turn out to be heavily regulated by this family of enzymes,” Keck notes. “That’s why bryostatin 1 can have such a wide range of biological effects. It’s not like a lot of agents that target one specific site in an enzyme and inhibit its activity. This is very different.”
Despite promising results as a cancer treatment in animal studies, bryostatin 1 has stalled in Phase II clinical trials. It has failed to show significant activity against tumors either on its own or in combination with other chemotherapeutic agents. “The bryostatins still haven’t quite found the right niche,” says Peter M. Blumberg, chief of the Molecular Mechanisms of Tumor Promotion Section at NCI. “By understanding the mechanism of the bryostatins we might be better able to pinpoint which are the specific subclasses of cancers in which this would represent the rational therapy.”
Although its status as a cancer-fighting agent may have taken a hit, bryostatin 1 has started to gain some traction in treating other diseases, particularly in illnesses associated with memory loss, such as Alzheimer’s disease and strokes.
Researchers led by Daniel Alkon, scientific director and professor at Blanchette Rockefeller Neurosciences Institute at West Virginia University, were trying to work out how memories are stored on the molecular level when they discovered that PKC plays a critical role in the process. “It’s a very powerful regulator of molecular switches that send signals, especially at the most important junctions in the brain called synaptic junctions—the connections in the brain between neurons,” he says. “We discovered when we form memories we actually induce the formation of new synapses, and that’s regulated by protein kinase C and a whole host of other molecular players in the orchestra that protein kinase C regulates.”
With this understanding, Alkon’s team wondered whether PKC might be relevant to the memory loss associated with Alzheimer’s disease. “It turns out that the central molecular pathways of the pathophysiology of Alzheimer’s disease all involve protein kinase C,” Alkon explains. This led Alkon to several compounds that activate PKC, of which bryostatin 1 was the most potent.
“We found that PKC activators are remarkably effective in animal models of Alzheimer’s disease in addressing virtually all of the aspects of Alzheimer’s disease,” Alkon says.
These compounds “enhance memory. They correct memory deficits. They restore lost synapses and prevent the loss of synapses. They prevent the death of neurons. They prevent the amyloid plaques. And they prevent the neurofibrillary tangles. All of those are hallmarks of Alzheimer’s disease,” Alkon continues. “There’s no one therapy except activators of protein kinase C that does that.” These findings, he argues, suggest a new way of looking at Alzheimer’s disease.
Animal tests with bryostatin 1 have also shown that it restores memory after strokes and traumatic brain injuries. “Essentially what it’s doing is building new connections in the brain and preventing the death of neurons,” Alkon says. “It also has the potential of enhancing memory in normal patients or aging patients or depressed patients. We believe that there is a tremendous potential here.”
Alkon recently received approval to begin a Phase II clinical trial using bryostatin 1 to treat Alzheimer’s. He wants to partner with a private-sector company before moving forward, however.
Bryostatin 1’s ability to activate PKC has also recently gotten attention for treating another disease—HIV. Patients with HIV who take the antiviral drug cocktails still retain latent reservoirs of the virus in their cells. That’s why the cocktails don’t cure the disease but merely treat it. Once a patient goes off the therapy the virus reawakens.
But PKC “can activate transcription factors that can rouse slumbering HIV proviruses,” according to Warner C. Greene, who directs virology and immunology research at the J. David Gladstone Institutes in San Francisco. “So bryostatin 1 is a drug that’s under active investigation for an eradication treatment,” he says, although he’s quick to point out that such therapy is still in the early stages. No animal testing has been done with bryostatin 1 and HIV, Greene notes.
Even if clinical tests prove the medicinal potential of bryostatins, treatments based on the compounds will have to grapple with supply. In the late 1990s, the now-defunct company CalBioMarine Technologies tried aquaculture, growing B. neritina on what Dominick Mendola, the company’s former president, describes as a giant “undersea box kite” off the California coast. Although they succeeded in growing the bryozoan, the company eventually went under as postponed clinical trials demolished demand for the bryostatin 1 it was ready to supply, and the firm was unable to secure venture capital funding in the early 2000s to stay afloat.
Bryostatin time line
1968
First samples of Bugula neritina screened for anticancer activity
1976
A compound that would come to be known as bryostatin 1 identified for the first time in extracts from B. neritina collected from the California coast
1982
Structure of bryostatin 1 reported
1990
First total synthesis of bryostatin 7 in 79 steps by Satoru Masamune and coworkers at Massachusetts Institute of Technology
1991
18 g of bryostatin 1 extracted from 14 tons of B. neritina collected off the California coast
1998
First total synthesis of bryostatin 2 in 72 steps by David A. Evans and coworkers at Harvard University
2000
First total synthesis of bryostatin 3 in 88 steps by Shigeru Nishiyama, Shosuke Yamamura, and coworkers at Japan’s Keio University
2008
First total synthesis of bryostatin 16 in 42 steps by Barry M. Trost and Guangbin Dong of Stanford University
2011
First total synthesis of bryostatin 1 in 58 steps by Gary E. Keck and coworkers of the University of Utah
First total synthesis of bryostatin 9 in 43 steps by Paul A. Wender and Adam J. Schrier of Stanford University
Total synthesis of bryostatin 7 in 36 steps by Michael J. Krische and coworkers at the University of Texas, Austin
The bryostatins, current research suggests, don’t actually come directly from B. neritina, but rather from a bacterial symbiont that lives within the bryozoan. The compound appears to protect the organism’s larvae from being eaten by predators. Scientists have tried to isolate the symbiont so that they might create bryostatins in a petri dish.
To date, however, no one has been able to culture the bacterium. “It may be missing some capabilities it needs to live outside of its host,” says Margo G. Haygood, a professor at Oregon Health & Science University who has been studying how the symbiont makes the bryostatin skeleton. She adds that, despite efforts, no one has been able to transfer enough of the symbiont’s biosynthetic machinery into another organism, such as Escherichia coli, to make the bryostatin skeleton.
And that has left the bryostatins’ fate in the hands of chemists. With their complex skeleton and multiple stereocenters, the bryostatins are a trophy for any synthetic organic chemist up to the challenge. Until recently, however, total syntheses of bryostatin natural products weighed in at more than 70 steps—too unwieldy to make large amounts of the molecules.
Advertisement
In 2008, Stanford University chemists Barry M. Trost and Guangbin Dong reported the synthesis of bryostatin 16 in only 42 steps (Nature, DOI: 10.1038/nature07543). And there’s been a flurry of activity in the field in 2011. A team led by the University of Utah’s Keck and graduate student Yam B. Poudel reported the first total synthesis of bryostatin 1—the one that’s been used in all the clinical trials—in 58 steps (J. Am. Chem. Soc., DOI: 10.1021/ja110198y). Paul A. Wender and graduate student Adam J. Schrier, also at Stanford, prepared bryostatin 9 in 43 steps (J. Am. Chem. Soc., DOI: 10.1021/ja203034k). And Michael J. Krische and coworkers at the University of Texas, Austin, set a new record for making the molecules when they prepared bryostatin 7 in just 36 steps (J. Am. Chem. Soc., DOI: 10.1021/ja205673e).
The recent syntheses are also highly convergent, with the longest linear sequence clocking in at 31 steps for Keck’s synthesis, 28 for Trost’s, 25 for Wender’s, and just 20 for Krische’s. And each shows off a different use of chemistry. Trost takes advantage of a palladium-catalyzed union of two alkynes to create the bryostatins’ macrocyclic structure. Keck makes use of a pyran annulation method to unite the A-ring and C-ring subunits with simultaneous formation of the B-ring. Wender uses a similar macrocyclization strategy, employing the Prins reaction to wed an aldehyde with a hydroxyallylsilane. Krische uses Keck’s assembly strategy but decreases the number of steps to make each fragment by employing hydrogenative carbon-carbon bond formation. Each strategy gives chemists flexibility to make a range of analogs.
“In natural product synthesis, I feel that it’s really important to select targets that represent authentic unmet challenges in terms of the chemistry and biology,” Krische says. “With sufficient resources, it’s pretty clear that one can complete the total synthesis of nearly any natural product. So I think now it’s incumbent upon synthetic organic chemists not only to make the target but to focus on how the target is made,” with an eye toward flexibility, he says. “It’s important to select natural products where the synthesis of the target is not an end point but a beginning.”
To that end, Krische says, his group is now aiming to use the synthetic methods they developed to make analogs of bryostatin in as few as 12 steps. He’s in good company in the bryostatin analog game. Wender has been making simplified versions of the bryostatins for 25 years, and Keck has been creating bryostatin analogs for the better part of the past decade.
“We need to understand collectively as a community that natural products are not made in nature to do what we ask of them. Bryostatin is not made in B. neritina for the purposes of addressing HIV or cancer or Alzheimer’s,” Wender says. “The natural product traditionally has been often perceived as being the drug, when in fact an emerging emphasis is that it’s a tremendous lead. And if we could learn what nature is teaching us in this lead, we could then, using modern science, translate that into molecules that would be more effective than what nature has produced.”
More than 100 bryostatin analogs—dubbed bryologs—have come out of Wender’s research group over the years. “We’ve been trying to understand the lesson of bryostatin and then to use what we have learned to come up with agents that are superior to the natural product,” he says.
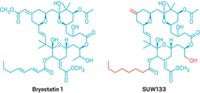
For example, they have learned that an alkoxy group at a certain position in the bryostatin backbone is critical. They’ve determined the structure of the C-ring and its surrounding functional groups are also important, as that’s the portion of the molecule thought to bind to PKC. Finally, they’ve figured out how to simplify bryostatin’s A- and B-rings , so the analogs maintain the same shape as the bryostatins but are easier to make.
Wender points to the analog from his lab known as “picolog” as one of the most promising. It can be made in fewer than 30 steps. It’s 100 times more potent than bryostatin 1 in some in vitro anticancer tests, and it’s shown promise in treating mice with leukemia.
While Wender sees potential new therapies from bryostatin analogs, Keck is more restrained. “Any talk of drugs based on bryostatin at this juncture is really premature because we don’t know yet what kinds of structures you need to elicit a particular kind of response,” he says. “What we’re doing is making a toolbox of compounds that vary in structure, and then going in and finding out in great and gory detail what those compounds do biologically. The goal is to link specific structural features with specific biological responses.”
The analogs made in Keck’s lab are known as Merle compounds, named after country music legend Merle Haggard, of whom Keck is a friend and “probably the world’s greatest fan.” Keck says his collaborator, NCI’s Blumberg, told him he needed succinct identifiers for his analogs that wouldn’t change from publication to publication. “I said, ‘I know just the thing. We’ll give them Merle numbers because nothing lifts my spirit like a Merle number,’” Keck recalls.
Keck believes the substitution around bryostatin 1’s A-ring is critical. His group, in collaboration with NCI’s Blumberg, compared analogs that were simplified around either both the A- and B-rings (Merle 23) or just the B-ring (Merle 28). Those that were simplified around the A-ring did not behave like bryostatin but instead behaved like the tumor-promoting phorbol esters. “This was a big surprise because these things look very much like bryostatin. They look nothing like phorbol esters, and yet to the cell, well, I guess the cell does not have ChemDraw,” Keck says .
“There’s a great opportunity to make important findings in biology just from looking at analogs that people are making,” Keck adds. “If nothing else, there’s a great deal to be learned about the fundamental biology that’s relevant to cancer, Alzheimer’s disease, and other diseases through this kind of research.”
So will one of the bryostatins or their analogs ever become a drug? It’s tough to say. “In my view there are two key things in advancing a natural product into drug development,” says Guy T. Carter, a consultant with natural products discovery consulting firm Carter-Bernan Consulting. “One is making enough material to start with, and the other is the ability to make a broad range of analogs in sufficient quantities in order to address whatever issues you encounter as you go through the development process,” such as problems with solubility, permeability, or metabolic stability.
Having a synthesis that lends itself to modifications makes the bryostatins attractive, but “it’s still going to be a hard sell,” Carter says. “I think the dogma in big pharma has always been that we don’t do total synthesis. It’s just not practical.” Still, he notes that some companies are challenging that dogma. He points to Eisai’s drug Halaven, which is an analog of a natural product made via a 62-step synthesis.
For the bryostatins ever to make it to patients will require a tremendous devotion of resources and a strong willingness on the part of those in charge to stick with such a project, Carter notes. “That bit of wisdom that’s required to see it through to the end product is something that is in short supply,” he says. “It’s much easier to say ‘no’ to something like that than to say ‘this is something special and therefore we need to devote the resources to make it happen.’ Pursuit of challenging targets like the bryostatins, while risky, has great potential for creating major breakthroughs in medicine and eventually profits for the company.”
John A. Lowe, a medicinal chemistry expert with the consulting firm JL3Pharma, doesn’t see pharma executives running out to make bryostatin analogs just yet, but notes: “It certainly is intriguing how much more approachable the bryostatins or their analogs are when you start talking about potential commercialization. It’s now competitive with the other things that are going on, and I don’t believe anybody believed that would be the case 20 years ago when the structures were elucidated. That in itself is pretty impressive.”











Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter