Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Hydrogenase Spills Secret
Crystallography reveals novel cluster behind oxygen tolerance, opening up new possibilities for fuel-cell applications
by Carmen Drahl
October 24, 2011
| A version of this story appeared in
Volume 89, Issue 43
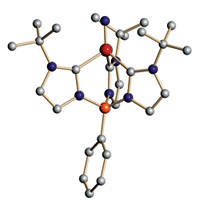
An enzyme that taps into hydrogen’s power with minimal interference from oxygen possesses a novel iron-sulfur cluster, according to two crystallography studies (Nature, DOI: 10.1038/nature10505 and 10.1038/nature10504). With nickel and iron clusters, hydrogenase enzymes split H2 into protons and electrons, but O2 squelches the activity of most hydrogenases. Some hydrogenases work in air, however, and potential applications such as H2-powered fuel cells or light-driven H2 production from water depend on uncovering the secret to those enzymes’ success. A German team led by Oliver Lenz of Humboldt University and Christian M. T. Spahn of Charité University Hospital, both in Berlin, and a Japanese team led by the University of Hyogo’s Yoshiki Higuchi each detected a cluster never before seen in an enzyme—four iron atoms and three sulfurs. These clusters typically have four iron and four sulfur atoms. The researchers propose that the distinctive cluster converts invading O2 to water by delivering electrons to the active site. Both studies showcase the versatility of iron-sulfur clusters, says Douglas C. Rees, who studies similar clusters at Caltech. “I would have loved to have done this work.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter