Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Switch-On Fluorescence
Biological Imaging: Functionalized nanoparticles light up upon entering cells
by Stephen K. Ritter
December 5, 2011
| A version of this story appeared in
Volume 89, Issue 49

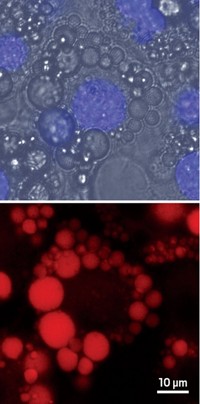
A research team based at Ireland’s University College Dublin has demonstrated fluorescence-switchable polymer nanoparticles in action. Bearing functional groups that turn on fluorescence for imaging when captured by cells, these particles are not subject to the interfering background fluorescence common with fluorophores that are always turned on.

According to Donal O’Shea, who spearheaded the work, the unique “off” to “on” switching “allows us to use the nanoparticles for real-time, continuous imaging of their uptake into live cells for the first time. Some of the movies we have recorded are quite dramatic.”
Near-infrared fluorescence imaging using molecular fluorophores is a popular method for investigating biological processes, such as the cellular uptake of molecules, including drugs. An often-encountered problem is background fluorescence from fluorophores not inside the cells. The extraneous light can mask imaging of events scientists want to see or limit the imaging to snapshots in time when the background fluorescence has been removed.
O’Shea’s team circumvented this problem by designing poly(styrene-co-methacrylic acid) nanoparticles covered with hydrophobic BF2-chelated azadipyrromethene groups (J. Am. Chem. Soc., DOI: 10.1021/ja208086e). These groups shy away from water, which causes them to aggregate, thereby compressing the fluorophores and quenching their fluorescence. Common surfactants or interactions with cellular components such as membrane phospholipids cause deaggregation. When the groups are apart, the fluorophores are free to cut loose and shine with their full fluorescence intensity.
To demonstrate the power of the fluorescence switching, the researchers tracked fluorescence after cellular uptake of the nanoparticles by human breast cancer and kidney cells. It takes about 15 minutes for a diffuse pattern of red fluorescence to emerge from the dark background as the particles enter cells and switch on. By 100 minutes, strong red fluorescence concentrates in individual cells. The particles don’t enter the nucleus, so that area in each cell remains dark.
Turn-on nanoparticles are “indeed a cool tool to follow fluorescence within living cells,” comments Wendelin J. Stark, a functional nanomaterials expert at the Swiss Federal Institute of Technology, Zurich (ETH). The inherent tendency of the new nanoparticles to quench when close to one another “is a different kind of switch for fluorophores,” Stark notes.
Besides biological imaging and drug delivery, the method “may also find interesting use in low-cost, portable detection of surfactants, maybe in water analysis,” Stark adds.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter