Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Why Quantum Dots Blink
Study uncovers two mechanisms that control spontaneous emission fluctuations
by Mitch Jacoby
December 12, 2011
| A version of this story appeared in
Volume 89, Issue 50
Random fluctuations in light emission from semiconductor nanocrystals, also known as quantum dots, are driven by two photoluminescence mechanisms, according to researchers at Los Alamos National Laboratory (LANL). The findings, which were revealed by a new spectroelectrochemical technique, uncover the causes of the “blinking,” a well-known phenomenon that limits the stability of quantum-dot-based devices such as solar cells and light-emitting diodes.
In addition to providing a foundation for developing a comprehensive theoretical model of this detrimental phenomenon, the study details an electrochemical method for suppressing it. The findings were presented at the Materials Research Society (MRS) meeting in Boston last month and were published in Nature (DOI: 10.1038/nature10569).
As methods to synthesize nanocrystals with well-controlled compositions and structures have abounded in recent years, the variety of applications that exploit nanocrystals’ optical and electronic properties have grown too. The list includes photovoltaic cells and other energy conversion devices as well as diodes, lasers, and other specialized light emitters. Quantum dots are also used for labeling and tracking cells and in other bioimaging applications.
TWINKLE TWINKLE
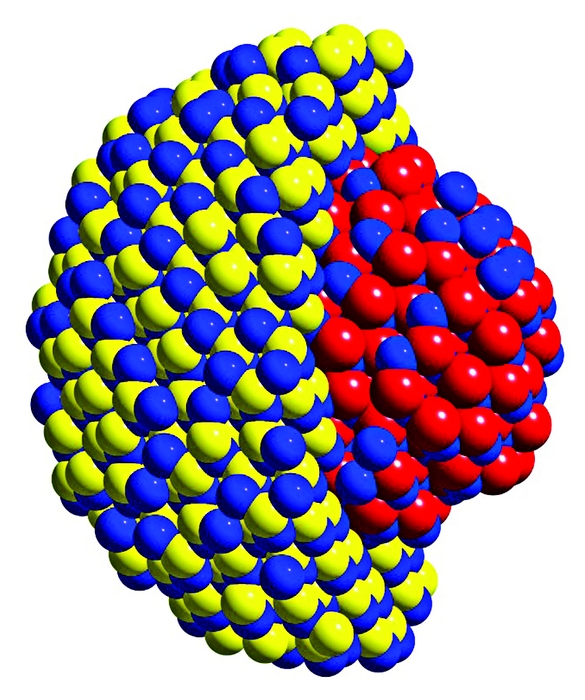

By applying a fl uorescence spectroscopy technique to CdSe-CdS core-shell nanocrystals (depicted in model), Galland (seen here with samples in hand) and coworkers at LANL have uncovered the mechanisms that cause the particles to blink.
Credit: Victor I. Klimov/LANL(both)
By applying a fluorescence spectroscopy technique to CdSe-CdS core-shell nanocrystals (depicted in model), Galland (seen here with samples in hand) and coworkers at LANL have uncovered the mechanisms that cause the particles to blink.
All of these uses for quantum dots are adversely affected by their tendency to blink. The underlying causes of that process have been the subject of lively debate because researchers have been unable to design telltale experiments that could pinpoint the origins of the blinking.
Now, Christophe Galland, Victor I. Klimov, and coworkers at LANL have done just that.
“We’ve developed a new spectroelectrochemical technique that allows us to study the effect of controlled charge injection on the intensity and lifetime of light emission from quantum dots,” Klimov said at the annual fall MRS meeting.
To get that information, the team probed the photoluminescence behavior of single quantum dots in solution by exciting the sample with a pulsed laser while they manipulated the charge state of the particles in a working electrochemical cell. For example, they gradually made the particles—CdSe-CdS core-shell nanocrystals—negatively charged (injected with electrons) by applying an increasingly negative potential to the solution, and then they analyzed the light emitted from the particles via microspectroscopy techniques.
By applying those methods to particles with a narrow range of shell thicknesses, the team determined that blinking is caused by two processes. One process results from light-driven charging and discharging of the nanocrystal core. After being irradiated, excited neutral quantum dots relax by emitting photons, which makes the particles appear bright. Charged particles appear dark because they relax by ejecting electrons in a process known as Auger electron emission.
“The majority of quantum dots, however, display blinking due to a different mechanism,” Klimov said. That process is based on charging and discharging of surface electron traps. If these traps are unoccupied, they can intercept energetic or “hot” electrons, thereby preventing the electrons from relaxing to a bright state—one that is characterized by photon emission.
Both blinking mechanisms can be controlled electrochemically, Klimov said, and applying an appropriate potential can completely suppress blinking.
“This study resolves the long-standing controversy concerning the origin of photoluminescence blinking,” says Alexander L. Efros, a Naval Research Laboratory theoretician who specializes in quantum dot physics. The LANL team showed that in addition to a “conventional” charging-discharging mechanism, a second process must be going on, he explains. “The interception mechanism that they propose is the only plausible explanation of these observations,” Efros says.
The team also showed that in nanocrystals with a thick shell, the interception mechanism is suppressed. That finding, Efros adds, “paves the way to designing nanostructures that will combine blinking suppression with extremely high photoluminescence yields.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter