Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Directed Evolution Helps Build Protein Containers
Chemists figure out how to engineer nature’s ministorage devices to stockpile more stuff
by Sarah Everts
February 7, 2011
| A version of this story appeared in
Volume 89, Issue 6
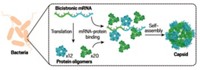
By using microbial evolution as a driving force, chemists have figured out how to coax the molecular boxes that nature uses as miniature storage devices into stockpiling more stuff (Science, DOI: 10.1126/sci ence.1199081). The development, by Donald Hilvert of the Swiss Federal Institute of Technology, Zurich, and coworkers, could improve the capacity and specificity of molecular containers that are used in fields as diverse as drug delivery and catalysis. The team engineered Escherichia coli cells to produce a protein called AaLS, which spontaneously forms icosahedral shells, or capsids. The researchers also engineered AaLS to have negatively charged residues to help it store away proteins that have a positively charged tag. They then provided the E. coli with a gene that codes for a protease enzyme that is toxic to the bacteria and that has the positive tag. Electrostatic attraction helps sequester the harmful protease in the capsid, but the bacteria also have an evolutionary impetus to improve the capsid’s ability to pack away the harmful protease. When the team exposed the E. coli to millions of randomly mutated capsid genes, the bacteria incorporated improved capsids that were five to 10 times better at impounding the protease.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter