Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
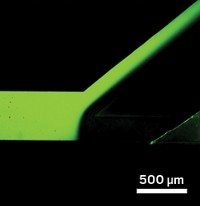
Miniaturized Membranes For Protein Separation
Microfluidics: A new isoelectric focusing method uses membranes to isolate specific proteins
by Rajendrani Mukhopadhyay
March 23, 2011
Proteomics researchers grapple with finding a certain protein in a veritable alphabet soup of others. Now scientists have devised a simple microfluidic method that can pull out specific proteins from a complex mixture (Anal. Chem., DOI: 10.1021/ac200073p).
Microfluidic characterization of proteins appeals to researchers because they can work with small amounts of their precious samples. But isolation of a protein from a sample "is always plaguing microfluidic analyses," explains bioengineer Greg Sommer at Sandia National Laboratories.
Researchers previously attempted to separate proteins by isoelectric focusing. Proteins flowed along in a solution with a pH gradient. When they hit the pH values at which they became neutrally charged, known as their pI values, they stopped moving. But because the method lacked a physical way to keep the isolated proteins in place, protein bands would bleed into each other.
Sommer’s team took a different tack: They downsized a membrane-based benchtop isoelectric focusing method. The researchers used polyacrylamide membranes with different pHs to separate the proteins.
When a protein mixture flowed down the microfluidic channel, the membranes trapped proteins based on their characteristic pI values. The separation took a few minutes; the benchtop method takes hours. Because the membranes kept the proteins separate, the investigators were able to capture clean samples for further characterization.
Using membranes with pH values between 5.3 and 6.5, the investigators could reproducibly separate model antibodies and enzymes dissolved in a simple buffer or within a bacterial cell lysate. Sommer says that they are now working on fine-tuning the pH values to improve the separation power of the method.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter