Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
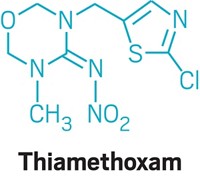
Taming Methyl Bromide
Pesticides: Researchers use a two-pronged strategy to prevent the fumigant from escaping into the environment
by Valerie Brown
March 2, 2011

Methyl bromide is a popular agricultural fumigant that can kill fungi, insects, weeds, nematodes, and bacteria. Unfortunately, it also depletes stratospheric ozone. Now researchers have devised a plastic film that reacts with or captures most methyl bromide before it enters the atmosphere (Environ. Sci. Technol. DOI: 10.1021/es103713k).
When planting crops such as strawberries, farmers fumigate their fields by injecting methyl bromide into soil. The United Nations' Montreal Protocol placed restrictions on methyl bromide, and the U.S. planned to phase it out by 2005. But because no substitute has such wide efficacy, exemptions allow methyl bromide use on certain crops.
Attempts to limit methyl bromide's escape into the environment have included applying plastic tarps to physically hold the fumigant in the soil, or applying a reactive compound to the soil to degrade the pesticide. But the two approaches had not been combined.
So Richeng Xuan, Scott Yates, and colleagues at the Department of Agriculture placed soil spiked with methyl bromide in a stainless steel container and covered it with a three-layer film. The film's outer layers were plastic. Its middle layer was tissue paper infused with an aqueous solution of ammonium thiosulfate. The chemical reacts quickly with methyl bromide to produce methyl thiosulfate and bromine, which Xuan says are easy to trap and remove.
The scientists found that after 72 hours at 40 °C, nearly all the methyl bromide had moved into the tissue paper and reacted with the ammonium thiosulfate. Only 0.15% of the methyl bromide seeped through all three layers. By contrast, 50% of the fumigant typically escapes to the atmosphere after application on agricultural fields.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter