Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Sensing Sugars On Proteins
Glycoprotein Analysis: Raman spectroscopy reveals a protein’s glycosylation state
by Laura Cassiday
August 11, 2011
When pharmaceutical companies produce protein-based drugs, they must ensure that the proteins have the proper sugar groups attached. A protein’s so-called glycosylation state can change its stability and function, as well as how the immune system reacts to it. Now, for the first time, researchers demonstrate that Raman spectroscopy can differentiate between the native and glycosylated forms of a protein (Anal. Chem., DOI: 10.1021/ac2012009).
A therapeutic antibody with the wrong sugars may lose its effectiveness or even cause a harmful immune reaction, says Roy Goodacre, a biological chemist at the University of Manchester, in the U.K. Currently, scientists most often characterize glycoproteins using mass spectrometry, he says. But the technique is time consuming and destroys the protein sample.
So Goodacre and his colleagues wanted to see if Raman spectroscopy could detect and quantify protein glycosylation. Chemists previously have used Raman to pinpoint structural features in proteins, such as β-strands. Companies could use the technique to analyze proteins during production without hurting their yields, he says.
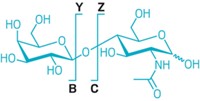
To test the principle, the researchers demonstrated that they could distinguish between the Raman spectra of RNase A and RNase B, the sugar-free and glycosylated forms, respectively, of bovine pancreatic ribonuclease, a well-characterized protein. When the scientists removed the sugar group from RNase B with chemical or enzymatic methods, the modified protein’s Raman spectrum resembled that of RNase A. By analyzing mixtures of RNase A and B at different relative concentrations, the researchers showed that they could quantify the fraction of protein that was glycosylated.
Goodacre now plans to test the method on well-characterized antibodies, with the hopes of eventually applying it therapeutic proteins.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter