Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Nanomedicines Stick To Cellulose
Nanoparticle Safety: Excreted nanoparticles containing drugs could pose a threat in the environment
by Leigh Krietsch Boerner
September 20, 2011

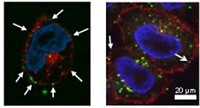
Nanomedicines are becoming increasingly popular. Some researchers worry, however, that these drugs encapsulated in nanoparticles may pass through the body, into sewage, and into the environment, where they may pose a threat to wildlife. Adding weight to such concerns, a new study finds that polymeric nanomedicines adsorb onto a surface commonly located in the environment (Langmuir, DOI: 10.1021/la202287k).
There are about 20 nanomedicines on the market today, according to Mustafa Akbulut, an assistant professor of chemical engineering at Texas A&M University. He estimates that about 110 more are in clinical or preclinical studies. But little is known about how nanomedicines behave in the environment. In mouse studies, researchers have found that some of these drugs get excreted in the urine. If the same is true in people, nanomedicines could get into groundwater and soils through sewer systems.
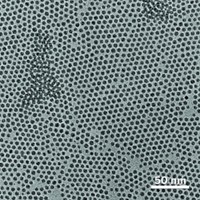
Akbulut wondered whether excreted nanomedicines might be adsorbed by the cellulose in plants, which in turn might be consumed by animals or bacteria. Researchers have previously shown that nanoparticles can increase in concentration as they move up the food chain, potentially causing toxicity to animals.
“Cellulose is the most abundant organic molecule in the environment,” Akbulut says. If nanoparticles made it into the environment, scientists would want to know how they would interact with plant cellulose.
In order to model how plants adsorb the excreted nanomedicines, graduate student Ming Zhang and Akbulut studied how polyethylene glycol nanoparticles filled with molecules of the painkiller ibuprofen were adsorbed and desorbed by a cellulose surface. Polyethylene glycol is commonly used in nanomedicine because it is one of the few polymer nanoparticles with Food & Drug Administration approval, Akbulut says.
Akbulut and Zhang created nanoparticles ranging from 46 to 271 nm in diameter. Using a technique called quartz crystal microbalance with dissipation, they then studied the rates of adsorption on cellulose. Akbulut and Zhang also looked at how fast the particles came off with a constant stream of water washing over the surface. Through atomic force microscopy, they found that smaller particles adsorb better onto cellulose. Larger particles slough off more readily.
However, this finding does not necessarily mean that nanomedicines should have a larger particle size, Akbulut says. Our bodies can only absorb particles around 300 nm in diameter or smaller.
There’s excitement about creating polyethylene glycol nanoparticles for medicine, says Stefan Franzen, professor of chemistry at North Carolina State University, but not enough work on their behavior and safety in the environment. Akbulut and Zhang’s study should open researchers’ eyes to some of the potential pitfalls of the technology, he suggests.
“It’s amazing to me that there aren’t more studies like this,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter