Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Magnetic Nanoparticle Rattles Tumors
Drug Delivery: Magnets steer drug-laden nanoparticles to tumors
by Erika Gebel
December 9, 2011

To increase the potency of anticancer drugs and protect healthy cells from the drugs’ toxic effects, researchers have developed a magnetic nanoparticle that directly delivers medication to tumors. In mice, these so-called nanorattles shrink tumors more than the drug alone does (Nano Lett., DOI: 10.1021/nl202949y).
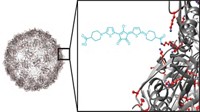
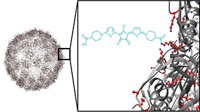
Other researchers previously had developed nanoparticles to deliver cancer drugs, says Galen Stucky of the University of California, Santa Barbara, but those systems didn’t allow researchers to easily move, and track, the particles through the body. To create a nanoparticle with those capabilities, Stucky and his colleagues designed a multi-layered nanoparticle that they could direct with a magnet and monitor using fluorescence imaging.
At the core of the particle is a silica-coated iron oxide magnet. Around this magnet, the researchers add a layer of yttrium oxide doped with ytterbium and erbium. These rare-earth elements are key to spotting the particles inside the body, says Stucky, because they fluoresce when excited by a laser. To create space inside the nanoparticle for a cargo of cancer-killing compounds, the chemists then convert the Y2O3 layer into a shell of NaYF4 by dunking the particles in a hot solution of HF and NaF for 2 hours. After this reaction, the nanoparticle resembles a baby’s rattle: The magnet isn’t attached to the outer shell and it can bounce around inside the shell.
To test the nanorattles, the researchers first loaded them with the anticancer drug doxorubicin by soaking them in a solution of the drug. They injected the doxorubicin-loaded nanorattles into the blood of mice with liver tumors grafted under their arms. The scientists guided the particles to the cancer cells by placing a strong magnet against the skin over the tumor. With fluorescence imaging, the chemists could spot the nanorattles assembled at the tumor site. The environments around cancer cells often have a lower pH than those near healthy cells, Stucky says, and that acidity stimulates the nanorattle to release its contents. After 21 days, the rattled tumors were 96% smaller than those in untreated mice. Meanwhile, tumors in mice given an equivalent dose of free doxorubicin were only 35% smaller than the control tumors.
“It’s a nice drug delivery platform,” says Jason Sakamoto of the Methodist Hospital Research Institute. He praises the experiments but stresses that further research should test the nanorattles’ effectiveness and safety. For example, he points out that little is known about the toxicity of rare-earth metals to humans. Stucky says they are currently testing the toxicity of nanorattles in human cells.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter