Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Taming Diazomethane
Organic Synthesis: Hazardous reagent generated without distillation
by Bethany Halford
March 26, 2012
| A version of this story appeared in
Volume 90, Issue 13

With the help of an inexpensive iron porphyrin catalyst, chemists in Switzerland have developed a safe way to generate diazomethane, an important reagent for cyclopropanation reactions (Science, DOI: 10.1126/science.1218781). The new user-friendly protocol provides chemists with easy access to cyclopropyl groups for pharmaceuticals and materials.
As tools of the chemical trade go, diazomethane (CH2N2) demands special care. Not only is it explosive, but it’s also highly toxic. Even less explosive analogs of the compound, such as trimethylsilyldiazomethane, have proven deadly, with two chemists succumbing to the effects of that compound in separate events in 2008 (C&EN, May 9, 2011, page 15).
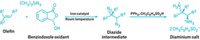
Chemists usually generate diazomethane by treating the N-nitroso compound Diazald with base and then distilling the resulting diazomethane with a specialized apparatus to lessen the chance of explosion. Now, Erick M. Carreira and Bill Morandi of the Swiss Federal Institute of Technology, Zurich, have developed a way of generating diazomethane in situ and then trapping its reactive carbene with a metal catalyst, circumventing the need for distillation.
The team slowly adds a Diazald derivative to 6 M potassium hydroxide. An iron porphyrin catalyst then consumes the resulting diazomethane by forming a metal-carbene complex. Next, this complex reacts with a double bond in another reagent to form a cyclopropyl group. The researchers found the reaction works well to cyclopropanate styrene derivatives, dienes, and enynes.
“The lack of practical, scalable methods for the construction of cyclopropanes is a long-standing problem in industrial chemistry,” notes Matthew M. Bio, director of process development at Amgen. He says the reaction “will certainly find application in pharmaceutical and fine chemicals manufacturing. This development has the potential to significantly reduce the cost of bringing new cyclopropane-bearing therapeutics and materials to market.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter