Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Watching Battery Anodes In Action
Electrochemistry: Microscopy reveals unusual phase transformation in high-capacity material
by Mitch Jacoby
March 26, 2012
| A version of this story appeared in
Volume 90, Issue 13

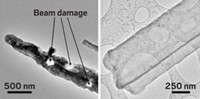
By zooming in on a nanosized lithium-ion battery with transmission electron microscopy (TEM), researchers have mapped out chemical and physical changes caused by charging and discharging the anode (Nano Lett., DOI: 10.1021/nl204559u). Through its details about electrochemically induced changes in the experimental anode material, the study may lead to strategies for designing low-cost batteries with greater capacity and longevity than today’s commercial Li-ion batteries.
The investigation, which took place at Pacific Northwest National Laboratory (PNNL), also broadens the capabilities of high-resolution microscopy techniques for examining batteries during charging and discharging—a technically challenging analytical problem that several research groups are pursuing.
Researchers think that swapping commercial Li-ion batteries’ carbon anodes with anodes made from silicon could boost battery capacity severalfold. That switch has not occurred, however, because the charging process—the electrochemical lithiation of silicon—causes Si anodes to swell to more than three times their initial size. That expansion, and the contraction that accompanies discharge, fatigues and cracks the anode material and leads to battery failure.
Nonetheless, silicon’s large charge capacity makes it an attractive anode candidate material with potential application in electric vehicles. So researchers in industry and academia continue to study its properties. For example, 3M just announced that it is matching a $4.6 million Department of Energy grant to develop Si-anode Li-ion batteries.
The PNNL team, which was led by Chongmin Wang and Fei Gao and included researchers at General Motors, Oak Ridge National Laboratory, and Applied Sciences, conducted its investigation on anodes made from a silicon-carbon nanocomposite. In non-TEM electrochemical tests, the group found that the potentially low cost material, which features hollow carbon nanofibers, remained stable through 100 charge-discharge cycles and exhibited a charge capacity more than five times as great as that of conventional carbon anodes.
On the basis of its TEM investigation coupled with density functional theory analysis, the team observed that as the silicon-coated carbon nanofibers were charged with lithium, the composite material initially formed an amorphous Li-Si film on the anode’s exterior. But as the charging process continued, the amorphous material suddenly adopted a crystalline structure with a Li-to-Si ratio of 15 to 4.
The researchers found that crystallization proceeded in an unusual manner. Upon lithiation, the material didn’t crystallize from a nucleation point, as typically occurs, but instead spontaneously and instantly crystallized as Li15Si4 when that ratio of elements was reached. Discharging the battery caused the crystalline material to revert to the amorphous form.
Wang says that although the electrochemical findings reported in this study might lead to better anode materials, as of now, “unfortunately, the material does not behave like a rubber band,” which can stretch without damage. The PNNL Li-Si anode material expands and contracts during cycling, he says, but does so more evenly and with less damage than pure silicon does.
Clare P. Grey of Cambridge University and the State University of New York, Stony Brook, says, “This is an interesting study that helps researchers understand why the crystalline but metastable silicide, Li15Si4, is formed instead of the thermodynamic phase, Li21Si5.” She adds that it shows how the in situ TEM method, in this case in combination with density functional theory, can be used to understand structure and morphological changes in battery materials.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter