Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Heck Reaction Goes Silyl
ACS Meeting News: Ligand enables silicon variant of familiar palladium-catalyzed chemistry
by Carmen Drahl
April 2, 2012
| A version of this story appeared in
Volume 90, Issue 14

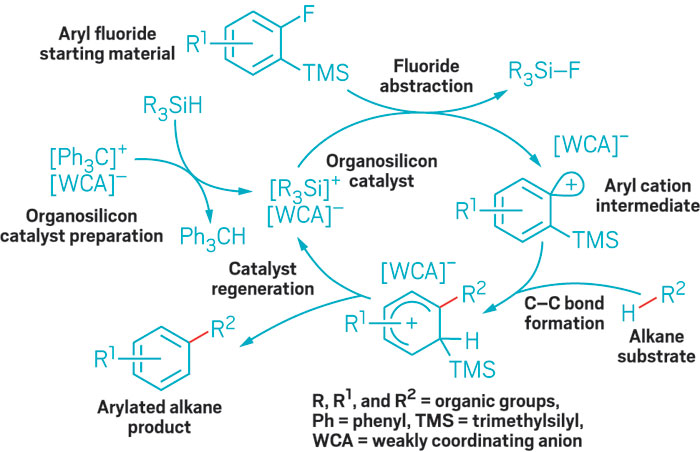
By designing a “goldilocks” catalyst—one with just the right balance of size and electron-donating properties—University of Delaware chemists have added a new reaction to the tool kit for forming carbon-silicon bonds.
The process converts alkenes, which are easily accessible starting materials, to vinylsilanes and allylsilanes, reagents with applications in several branches of synthesis. Donald A. Watson, who led the research, presented the work last week at the American Chemical Society’s national meeting in San Diego.
Chemists already have ways of obtaining allylsilanes and vinylsilanes, “but they’re not easy to make, and they tend to require harsh conditions,” Watson told C&EN. Building on the work of other researchers, Watson, graduate students Jesse R. McAtee and Sara E. S. Martin, and colleagues set out to develop a mild, palladium-catalyzed reaction that would convert the C–H bond of an alkene to a C–Si bond, analogous to the Nobel Prize-winning Heck reaction, which converts those C–H bonds to C–C bonds.
The group’s first attempts didn’t work, but they eventually realized that the problem was a bulky ligand on their catalyst. It provided much-needed electron donation, but it just got in the way, preventing the reaction, Watson explained. Eventually, his group found a ligand that was not too big and not too small: tert-butyldiphenylphosphine. With their ligand now just right, they modified the reaction conditions to use the silane source chlorotrimethylsilane in place of iodotrimethylsilane because the former sells for a fraction of the price (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201200060).
What’s most interesting about the new method is that it generates a trimethylsilylmetal intermediate that could be broadly useful, Scott E. Denmark, an expert in organosilicon chemistry at the University of Illinois, Urbana-Champaign, told C&EN. Although the compounds the team reported can be made in other ways, the researchers “have an opportunity for making an impact on other less readily available silanes,” he said.
It’s possible the method could even be applied to polymers such as silicones, added Michael A. Brook, an expert in materials chemistry of silicon at McMaster University, in Ontario.
In San Diego, Watson mentioned that his team is working toward an X-ray structure of their trimethylsilylmetal intermediate, which he said will help them further optimize reaction conditions.
“This is a creative piece of work,” said Edward A. Anderson, who studies vinylsilanes at Oxford University. He cautions that the reaction’s current need for excess silane might limit its application to highly functionalized substrates. However, the Delaware team’s search for the best ligand “showed great insight,” he added. “It is surprising that this reaction has not been developed before now.”
C&EN Covers The ACS National Meeting
Want the scoop on the ACS meeting in San Diego? Check out C&EN Picks, a series of videos that spotlight sessions selected by C&EN staff. Reporters also fan out across the meeting to bring you news coverage. Find it all collected at C&EN's meeting page, cenatacs.tumblr.com.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter