Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Another Form Of Oxygen
Computational chemistry: Model predicts insulating spiral-chain O4 structure at high pressures
by Celia Henry Arnaud
January 9, 2012
| A version of this story appeared in
Volume 90, Issue 2

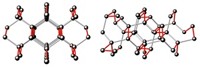
At pressures of nearly 2 terapascals (TPa), molecular oxygen forms a spiral-chain O4 structure with unusual properties, a computational study predicts (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1119375109).
Yanming Ma of Jilin University, in Changchun, China; Ho-kwang Mao of the Carnegie Institution of Washington; and coworkers used a computational method known as CALYPSO (crystal structure analysis by particle-swarm optimization) to search for structures of oxygen. In the past, this method has predicted the structures of known systems.
The researchers performed structural simulations over the pressure range of 0.02–2.0 TPa. The simulations predicted a previously observed molecular O8 structure, which is stable over the pressure range 0.008–1.92 TPa. At 2 TPa, they discovered the previously unknown O4 structure, which forms a square spiral chain with four oxygen atoms per turn. The structure is similar to sulfur’s high-pressure S4 phase.
“This spiral-chain form is the first monatomic form of oxygen. The oxygen atom is the basic constituent unit of the solid,” Ma says. “All other known forms of oxygen exist in the molecular O2 form where the basic constituent unit is the O2 molecule.”
The researchers were surprised to find that the new O4 structure is an insulator rather than a superconductor, like the low-pressure metallic O8 phase.
“Oxygen metallizes at the much lower pressure of 96 GPa, and at that pressure it looks like any other shiny metal,” says Malcolm I. McMahon, deputy director of the Center for Science at Extreme Conditions at the University of Edinburgh, in Scotland. “The conventional wisdom has long been that the application of high pressures metallizes materials. But predictions like this are now suggesting that transitions back to insulating forms may take place at extreme compression.”
Confirming such a structure “would be an extremely challenging experiment,” McMahon says, but with dynamic compression techniques such as those available at the National Ignition Facility at Lawrence Livermore National Laboratory, “it is no longer impossible.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter