Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Speedy Tool For Crystallography
Structural Biochemistry: Serial femtosecond crystallography achieves near-atomic resolution
by Stu Borman
June 4, 2012
| A version of this story appeared in
Volume 90, Issue 23

Researchers have shown for the first time that a recently developed technique called serial femtosecond crystallography (SFX) can take structural snapshots of proteins with near-atomic resolution.
SFX uses a free-electron laser (FEL) beam of high-energy, short-duration X-ray bursts to determine structures. It can obtain structures from microcrystals that are too small for analysis by conventional crystallographic instruments. SFX could thus make it possible to reveal the high-resolution structures of many biomolecules that have resisted traditional crystallographic analysis. Crystal structures are vital tools for designing drugs, studying how biomolecules interact, and determining enzyme mechanisms.
The SFX concept was proposed in 2000 by crystallographer Janos Hajdu of Uppsala University, in Sweden, and coworkers (Nature, DOI: 10.1038/35021099). SFX was used last year to determine protein structures, but the low-energy X-ray beam in that study permitted only correspondingly low resolution—about 8 Å, far above the approximately 3-Å threshold at which atoms can begin to be distinguished (C&EN, Feb. 7, 2011, page 27).
The advent of higher energy FEL X-ray beams has now made it possible for scientists to use SFX to obtain a 1.9-Å structure, approaching full atomic resolution, from lysozyme microcrystals too small for conventional crystallography. The work was carried out by Sébastien Boutet of SLAC National Accelerator Laboratory, Menlo Park, Calif., and 53 other scientists from Germany, the Netherlands, France, Sweden, and the U.S. (Science, DOI: 10.1126/science.1217737).
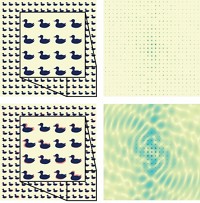
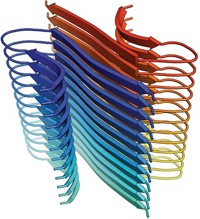
To obtain the structure, the team focused an FEL X-ray beam and directed it through a liquid-jet stream containing lysozyme microcrystals in random orientations. A sophisticated X-ray array detector captured diffraction patterns of the crystals in different positions, and the patterns were assembled into a 3-D structure.
With the 5- to 40-femtosecond X-ray pulses used in the study, “the idea is to outrun radiation damage, even though eventually the sample is completely vaporized” by the high-energy X-rays, Boutet says. X-rays travel through the sample at the speed of light, whereas damage propagates at slower speeds.
“It is breakthrough work with very high potential for biology, materials science, and condensed-matter physics,” FEL expert Jochen R. Schneider of DESY, in Hamburg, Germany, says of the study.
Hajdu comments that “SFX opens a new window for structural studies.” Microcrystals can often be obtained from difficult-to-crystallize proteins, “and SFX now gives you the chance to see if they diffract or not.” Hajdu and others believe SFX with still-higher-energy FEL X-rays could also make it possible to structurally analyze biomolecules without having to crystallize them at all and to make molecular movies of reactions.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter