Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Boron Wrangled Into Stable Triple Bond, Four-Atom Chain
Main-Group Chemistry: Exotic species represent new forays into boron bonding
by Bethany Halford
June 18, 2012
| A version of this story appeared in
Volume 90, Issue 25
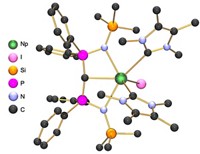
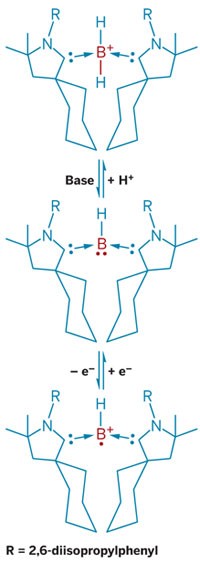
Chemists seeking strange new structures from the main group of elements have bagged two long-sought species with boron at their cores. Researchers in Germany report the synthesis of the world’s first boron-boron triple bond that’s stable at ambient temperatures (Science, DOI: 10.1126/science.1221138) as well as a four-boron chain held together by sp2 bonds (Nat. Chem., DOI: 10.1038/nchem.1379).
“Due to its inherent electron deficiency, boron is a very particular element, which behaves completely differently from its neighbor carbon,” says Holger Braunschweig, the University of Würzburg chemist who spearheaded the efforts to create these new species. “We show for the first time that under the right conditions boron can adopt two typical bonding patterns of carbon. Presumably, this will have major implications for future work on boron-containing molecules and, in particular, boron-containing materials,” he says.
The key to getting boron to behave more like carbon, Braunschweig says, was to stabilize the atom with the appropriate groups. In the case of boryne, the researchers used two bulky N-heterocyclic carbenes. For the tetraboron chain, they appropriated an iron atom.
The synthesis of a stable boryne “shows, once again, the unique capabilities of the determined synthetic chemist,” comments Gregory H. Robinson, a chemist at the University of Georgia who specializes in making exotic triple bonds. The new species, he says, “shows that main-group chemistry is alive and well.”
Next, Braunschweig’s team plans to see how the B≤B compound behaves in reactions with small, unreactive molecules that are usually activated only by transition metals. “Expectations are high that we will reveal highly unusual reactivity patterns here,” Braunschweig says.
As for the boron chain, he hopes to create even longer metal-stabilized boron oligomers. “Such boron chains are supposed to have highly interesting electronic properties and could, in the long term, establish a new class of molecular boron-containing materials.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter