Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Method Better Pins Down Antibodies
Immobilization strategy orients antibodies without blocking or altering antigen binding sites
by Celia Henry Arnaud
June 25, 2012
| A version of this story appeared in
Volume 90, Issue 26
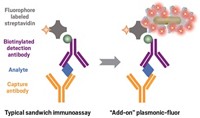
A new immobilization strategy attaches antibodies to surfaces in an oriented fashion without blocking or altering antigen binding sites, according to scientists at the University of Notre Dame (Langmuir, DOI: 10.1021/la301887s). Standard methods for immobilizing antibodies can result in randomly oriented antibodies, many of which are in inactive forms. In the new method, antibodies are uniformly oriented on the surface and their antigen binding site remains unaltered. Başar Bilgiçer and coworkers immobilized antibodies on detector surfaces using a photochemical method in which indole-3-butyric acid conjugated to the detector surface serves as a cross-link to the antibody’s nucleotide binding site, a conserved region found in the variable domain of all antibody classes. Upon exposure to UV light, aromatic side chains in the binding site form radicals that covalently bond with indole-3-butyric acid. Immunoassays performed with antibodies immobilized in this way were more efficient and more sensitive than assays with antibodies immobilized by physical adsorption or lysine conjugation. The immobilization strategy did not, however, decrease the limit of detection, the researchers note.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter