Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Bringing HIV Out Of Hiding
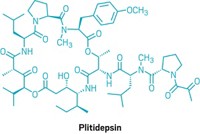
Drug Discovery: Bryostatin analogs fight dormant HIV, may be key to HIV/AIDS cure
by Stu Borman
July 16, 2012
| A version of this story appeared in
Volume 90, Issue 29

The synthesis of analogs of a bryostatin natural product could advance the eradication of AIDS by ferreting HIV out of its hiding places in immune cells.
Highly active antiretroviral therapy (HAART), the current standard for HIV drug treatment, fights the virus by attacking it in multiple ways simultaneously. But HAART drugs are toxic and attack only the active virus. People infected with HIV must take the drugs for life because of HIV’s latency—its tendency to adopt a dormant provirus form in immune cells, from which the virus emerges over time to reestablish active infection. If HIV latency could be eliminated, HAART could actually cure patients.
Certain natural products can bring the provirus out of dormancy. Obtained from the bark of a Samoan tree, the natural product prostratin is being considered for clinical testing even though its potency in activating latent HIV is low.
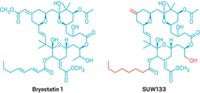
The natural product bryostatin 1 has similar activity and about 1,000 times the potency of prostratin. Coming from an aquatic invertebrate, it is difficult to obtain and hence expensive. Bryostatin 1 also causes side effects such as muscle pain.
Now, Jerome A. Zack, codirector of the UCLA AIDS Institute; Paul A. Wender, a synthetic organic chemist at Stanford University; and coworkers report the synthesis of promising analogs. In vitro tests show that the analogs, dubbed “bryologs, ” are at least as potent as bryostatin 1 in activating dormant HIV (Nat. Chem., DOI: 10.1038/nchem.1395). They are also readily accessible by synthesis, easily modifiable, and seemingly nontoxic. Studies of the bryologs in an animal model are in progress.
The work is “a significant accomplishment, since prostratin is too impotent,” says Douglas D. Richman, director of the UC San Diego Center for AIDS Research. The bryologs are “a promising class of drugs for activating the latent HIV reservoir, but animal and clinical confirmation of the agents’ activity is still needed,” he says.
“The new bryologs are an important discovery, and I am intrigued with their improved potency,” says Warner C. Greene, director of the Gladstone Institute of Virology & Immunology at UC San Francisco. “They could well become part of a cocktail of drugs. Of course, two important issues are whether they will synergize with other agents and have acceptable toxicity profiles.”
“The tour de force complex synthesis of bryologs is a brilliant realization of the goals of function-oriented synthesis,” says synthetic chemist Erick M. Carreira of the Swiss Federal Institute of Technology, Zurich. “This strategy brings the viral terrorists out of hiding, where they can be targeted for destruction. The combination of the new bryologs with other current antiretroviral therapies is highly promising and offers new hope for treatment.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter