Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Flexible Porous Capsules Catalyze Reaction
Molybdenum centers in metal-based porous structures serve as active sites
by Stu Borman
August 6, 2012
| A version of this story appeared in
Volume 90, Issue 32
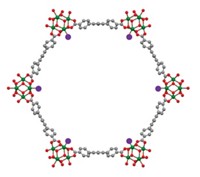
A few years ago, Ira A. Weinstock of Ben-Gurion University of the Negev, in Israel, and coworkers showed that the flexible pores of molybdenum oxide-based capsules could adjust to accommodate unexpectedly large “guest” molecules (C&EN, April 6, 2009, page 26). They had speculated that the capsules—discovered by Achim Müller of the University of Bielefeld, in Germany—might be useful for promoting reactions. They have now achieved that goal for the reversible catalytic cleavage and reformation of methyl tert-butyl ether in water (J. Am. Chem. Soc., DOI: 10.1021/ja304513t). They attribute the catalytic activity to the availability in each of the capsules of 60 cationic molybdenum(V) centers, which become acidic and are activated catalytically when acetate ligands bound to the centers are controllably replaced by water ligands. The controllability makes it possible to tune the capsules’ catalytic power. Weinstock notes that the active metal cations could also be used as substrate-binding sites to catalyze regio- and enantioselective C–C bond-forming reactions and that the capsules “present new possibilities for investigating the effects of nanoconfinement on catalysis in water.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter