Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Ordered Amorphous Carbon Clusters
Solvent molecules keep crushed fullerenes in line, creating a crystalline material from amorphous building blocks
by Stephen K. Ritter
August 20, 2012
| A version of this story appeared in
Volume 90, Issue 34
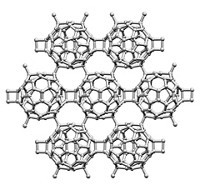
By squeezing the daylights out of solvated C60 fullerenes, materials scientists have created a hybrid material that maintains a long-range ordered lattice structure like that of a crystal even though its fundamental building blocks are amorphous carbon clusters (Science, DOI: 10.1126/science.1220522). Atomic structure is typically based on short-range order for liquids and amorphous solids or long-range repeating patterns for crystalline materials—but not both at the same time. A research team led by Lin Wang of the Carnegie Institution of Washington compressed C60 fullerene molecules solvated with xylene molecules in a diamond anvil cell. Using a combination of X-ray and other spectroscopic techniques, in conjunction with large-scale computer simulations carried out by Xiao Cheng Zeng’s group at the University of Nebraska, Lincoln, the researchers found that when the pressure reaches 32 gigapascal, or about 316,000 atm, the fullerene spheres collapse to form amorphous clusters separated by xylene molecules. The xylene molecules hold steady, keeping the carbon clusters aligned to maintain long-range crystalline order. Like other forms of carbon, the new material is anticipated to exhibit a range of mechanical and electronic properties, depending on the ratio of fullerene molecules to solvent molecules, the type of solvent used, and the addition of metal atom dopants.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter