Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists Score Alkyl Fluorination
Organic Synthesis: Simple, direct fluorination of C–H bonds adds to research hot streak
by Stephen K. Ritter
September 17, 2012
| A version of this story appeared in
Volume 90, Issue 38
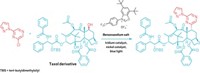
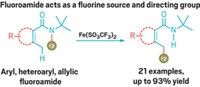
Chemists now have a general method to directly fluorinate alkyl C–H bonds, thanks to work by Princeton University’s John T. Groves and coworkers (Science, DOI: 10.1126/science.1222327). The operationally simple reaction features a manganese porphyrin catalyst and easily handled fluoride salts as the fluorine source. It allows difficult fluorinations under mild reaction conditions at previously inaccessible sites on an array of aliphatic molecules of medicinal importance.
“This is really powerful chemistry that will be super useful in both academic and industrial sectors,” comments Phil S. Baran of Scripps Research Institute. “It is a mechanistically intriguing transformation that has been on many people’s wish list for years.”
Fluorine chemistry has been on a hot streak during the past five years, with researchers cranking out one paper after another describing advances in organic synthesis (C&EN, Feb. 27, page 10). Even in this context, the new reaction by Groves and coworkers is advantageous because it works on substrates other than aromatic or unsaturated compounds and doesn’t involve cross-coupling or tricky organofluorine reagents. The reaction is also unusual in that the catalyst is not a traditional transition-metal complex.
Groves tells C&EN his group was inspired by its 2010 discovery of an aliphatic chlorination reaction using a manganese porphyrin as the catalyst and hypochlorite ion (OCl–) as the chlorine source (J. Am. Chem. Soc., DOI: 10.1021/ja105548x). Groves says this reaction got him thinking that analogous—but much more difficult—aliphatic fluorination might be possible if the right fluoride source could be found and a key manganese oxo-fluoride intermediate could be generated.
The researchers discovered that a combination of silver fluoride and tetrabutylammonium fluoride trihydrate works as a fluoride source, and oxidizing the manganese porphyrin with iodosylbenzene can generate the oxo complex. In their proposed mechanism, worked out with computational chemist William A. Goddard III and coworkers at Caltech, the starting Mn(III) porphyrin chloride is fluorinated and then oxidized to form the Mn(V) oxo-fluoride species. The oxo complex abstracts an alkyl hydrogen atom from the substrate to create an alkyl radical. That radical later unites with a fluoride from a Mn(IV) difluoride species—considered the active catalyst—to form the fluorinated product with surprising efficiency.
The reaction works with alkanes, terpenoids, and steroids in 50 to 60% yields. It’s also tolerant of common functional groups, Groves says, which is helpful in late-stage fluorination steps when a drug candidate is already loaded with other functional groups.
The new method “is a quantum leap in fluorination,” says Harvard University’s Tobias Ritter. “The reaction will serve as a stepping-stone for applications in several fields.” For example, the low cost of fluoride salts may enable new economically viable methods for large-scale manufacture of commodity fluorochemicals, he says. In addition, using 18F in the synthesis could quickly access new labeled molecules for positron emission tomography imaging.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter