Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Neutron Structure Of Human Enzyme-Drug Complex
Neutrons, unlike X-rays, visualize hydrogens and charge states
by Stu Borman
September 24, 2012
| A version of this story appeared in
Volume 90, Issue 39
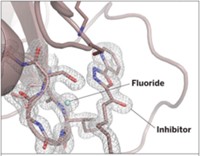
Researchers have used neutron crystallography for the first time to determine the structure of a human enzyme, carbonic anhydrase, bound to a clinical drug, acetazolamide (J. Am. Chem. Soc., DOI: 10.1021/ja3068098). Robert McKenna of the University of Florida and coworkers found that neutron crystallography makes it possible to visualize hydrogens (or deuteriums), hydrogen bonding, and charge states—molecular features that are not visible with conventional X-ray crystallography and are potentially valuable for rational drug design. Neutron crystallography has been around for decades, and its use to structurally analyze two enzyme-ligand complexes has been reported—but in one case the enzyme wasn’t human and in the other the ligand wasn’t a current drug. Carbonic anhydrase expert Claudiu T. Supuran of the University of Florence, in Italy, comments that the study resolves a controversy about the protonation state of carbonic anhydrase-bound acetazolamide, reveals previously unobserved H-bonding interactions that may help explain the agent’s high activity with some forms of the enzyme, and could be “of great help for the drug design of acetazolamide-like compounds.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter