Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Nanoparticles Boost Drug Solubility
BASF, Harvard University collaboration leads to promising nanoscale drug formulations
by Amanda Yarnell
January 23, 2012
| A version of this story appeared in
Volume 90, Issue 4

For drug compounds trying to make their way from the lab to the market, poor solubility is often the kiss of death. It’s one of the most common reasons for a drug candidate to drop out of the development pipeline.
And it’s only going to get worse, according to industry observers. Active pharmaceutical ingredients entering the pipeline are larger and more lipophilic than ever before.
“Seventy percent of new active pharmaceutical ingredients fail in development because of poor solubility and therefore bioavailability,” according to Andreas Kreimeyer, BASF’s executive director for research. BASF is betting that nanoformulation technology it’s developing in collaboration with Harvard University may improve those odds.
Kreimeyer spoke at a meeting in November that brought together dozens of BASF scientists with colleagues at Harvard, as well as many others developing nanoscale formulations to improve drug solubility.
BASF and Harvard have been working together on drug nanoformulations since BASF funded the BASF Advanced Research Initiative at Harvard in 2007. BASF is hoping the five-year, $20 million collaboration will lead to nanoscale technologies that significantly boost the solubility of drug candidates.
Nanoformulations is one of the initiative’s three thrusts, the others being antimicrobial surfaces and microfluidics. Already, the initiative has yielded 10 patents and more than 20 publications. But “time to market” remains the most important factor in such university collaborations, Kreimeyer said at November’s meeting.
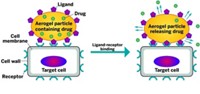
In a talk that kicked off the meeting, Harvard chemistry professor George M. Whitesides pointed to Celgene’s Abraxane as an example of how a nanoformulation can boost a drug’s bioavailability. The injectable consists of the poorly soluble anticancer drug paclitaxel bound to nanoparticles built from the protein albumin. The human body relies on this protein to carry molecules that are poorly soluble in water.
Scientists from various institutions described similar nanoscale protein-, polymer-, and lipid-based carriers that might be tethered to a drug to boost solubility and thus bioavailability. The meeting also showcased the various nanoformulation strategies that BASF is pursuing in its collaboration with Harvard.
A successful nanoformulation strategy could be applied to many different small-molecule drugs, said Jens Rieger, senior vice president of BASF’s advanced materials and systems research division. The key, he explained, is nanostructures’ high surface area, which boosts their solubility.
What’s more, when confined to the nanoscale, a solid drug may not fully crystallize, added David Weitz, a professor in Harvard’s School of Engineering & Applied Sciences. Instead it can remain amorphous, which further boosts its solubility.
One of the strategies scientists at BASF and Harvard are examining involves formulating small-molecule drugs as nanostructured foams. BASF principal scientist Sebastian Koltzenburg reported that his team has made such foams by saturating a solid solution containing the antifungal itraconazole with supercritical carbon dioxide. Rapidly decreasing the pressure results in a nanostructured foam that dissolves far more quickly than the drug normally does.
“It’s sort of analogous to how we make insulation foams,” Koltzenburg said. The team is now making similar foams with other active pharmaceutical ingredients.
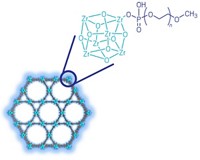
BASF and Harvard scientists are also “nanotemplating” small-molecule drugs using arrays of inorganic nanoparticles. In one experiment, the scientists filled the spaces in between densely packed SiO2 or CaCO3 nanoparticles with fenofibrate, a cholesterol-lowering drug that suffers from low solubility.
The inorganic nanoparticle template can be removed with hydrofluoric acid, leaving behind a nanostructured pure drug that dissolves readily in water. But this harsh etching step can be sidestepped, Koltzenburg noted. The composites burst when exposed to gastric fluids, releasing the drug as readily soluble nanoparticles.
Another strategy the scientists are investigating is whether a hydrophobic drug can be coprecipitated with a CaCO3 carrier to give an intercalated nanostructure that rapidly disperses in the human gut. They’ve managed to make these nanostructured hybrid materials from the steroid danazol and the seizure drug carbamazepine, both of which are poorly soluble.

The meeting also showcased strategies for nanostructuring pure drugs under development outside the walls of BASF and Harvard. For example, Joseph DeSimone, a chemistry professor at the University of North Carolina, Chapel Hill, described a method for molding nanoparticles of pure small-molecule drugs. The strategy relies on his particle replication in nonwetting templates (PRINT) technology, which creates nanoparticles with defined shapes using perfluoropolyether molds.
North Carolina-based Liquidia Technologies is now scaling up the PRINT process for making pure drug nanoparticles. It believes the strategy could be applied to many different pharmaceutical classes.
But not everyone believes that nanoformulations will prove a one-size-fits-all solution for solubility. “Nanoformulations have been called a universal approach,” said Thomas Rades, chair of pharmaceutical sciences at New Zealand’s University of Otago. “But they’re not a panacea.” He noted that their long processing times can pose drug stability hurdles, and quality control can be a challenge.
Still, BASF is hopeful the technologies it is developing in collaboration with Harvard might be a solution for many of the small molecules that are cast off during development because of solubility issues, said Martin Widmann, senior vice president of BASF’s pharmaceutical ingredients and services business unit. “These technologies could help pull many different discarded compounds out of the drawer.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter