Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Copper Continues To Advance Trifluoromethylation
Two groups report new copper-catalyzed reactions for adding trifluoromethyl groups to organic substrates
by Stephen K. Ritter
October 1, 2012
| A version of this story appeared in
Volume 90, Issue 40
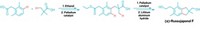
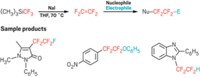
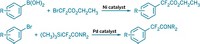
Copper-promoted trifluoromethylation reactions have been successful during the past three years, and researchers continue to broaden the scope of the chemistry and streamline procedures. Vladimir V. Grushin and coworkers at the Institute of Chemical Research of Catalonia, in Spain, now report a new synthesis of α-trifluoromethylated ketones (J. Am. Chem. Soc., DOI: 10.1021/ja307783w). They used CuCF3, which they make from CuCl and fluoroform (CHF3), a process Grushin’s group invented last year. Fluoroform is an inexpensive side product of polytetrafluoroethylene production, and the ability to use it in trifluoromethylations has opened the way to affordable large-scale use of CuCF3. Grushin’s team previously used CuCF3 to trifluoromethylate aryl halides and aryl boronic acids. In the current work, the researchers synthesized two dozen α-trifluoromethylated aryl and aliphatic ketones. In other CuCF3 chemistry, Melanie S. Sanford and coworkers at the University of Michigan report a mild, easy method for trifluoromethylation of aryl boronic acids (Org. Lett., DOI: 10.1021/ol3022726). They used CuCl with NaSO2CF3 (Langlois’ reagent) and tert-butyl hydroperoxide, which react in situ under ambient conditions to create trifluoromethyl radicals. Sanford’s team used the system to prepare a range of easily purified aryl and heteroaryl trifluoromethyl derivatives.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter