Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Synthesis Via Electrospray
Ion source typically used for mass spectrometry speeds up carbon-carbon bond-forming reactions
by Bethany Halford
October 15, 2012
| A version of this story appeared in
Volume 90, Issue 42
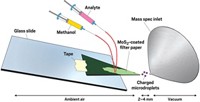
Electrospray—a mainstay of mass spectrometrists in which an electric field is used to disperse a solution into a fine mist of charged droplets—might also find a place in synthetic chemists’ tool kits, according to a report (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201206632). Compartmentalized liquids such as micelles and emulsions are often used to enhance chemical reactions. Thomas Müller of the University of Innsbruck, in Austria, along with Abraham Badu-Tawiah and R. Graham Cooks of Purdue University wondered whether the charged microdroplets generated with electrospray might serve the same purpose. The chemists studied the base-catalyzed Claisen-Schmidt condensation of 1-indanone and 4-chlorobenzaldehyde. They conducted the reaction in a flask at room temperature, as well as in an electrospray apparatus equipped with four multiplexed electrosonic spray ionization tips. Whereas the benchtop reaction took several hours to complete, the electrospray method generated products in better than 90% yield within the time of flight of the evaporating charged droplets. The technique, the researchers note, could be used to make milligram quantities of material in a matter of minutes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter