Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Light Can Drive Ullmann Reaction
Photochemistry: New mechanistic insight into century-old C–N bond formation process
by Elizabeth K. Wilson
November 5, 2012
| A version of this story appeared in
Volume 90, Issue 45

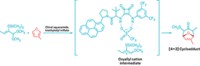
Light exposure can trigger the seminal, 110-year-old Ullmann reaction—which typically forges C–N bonds via heat and a copper complex—showing that the reaction can proceed via single-electron transfer (Science, DOI: 10.1126/science.1226458). This finding will prompt the design of new ways to leverage this type of coupling reaction, which is widely used to form arylamines from aryl halides and amines for use in pharmaceuticals and materials science.
“Now we can design reaction schemes that use the single-electron transfer pathway in Ullmann C–N coupling, and maybe other types of reactions,” says Jonas C. Peters, who performed the study in collaboration with Gregory C. Fu and other colleagues at California Institute of Technology. The work may lead to practical variants of the C–N bond-forming process that work under mild conditions, he says.
“It is definitely a major breakthrough in the field of copper catalysis and will undoubtedly break new ground,” says Gwilherm Evano of the Université libre de Bruxelles, in Belgium, who develops natural product syntheses using copper catalysts.
UCLA chemistry professor Kendall N. Houk, whose computational studies of the reaction suggested the possibility of a single-electron transfer mechanism, says the new experimental work is “a major advance in a venerable reaction. Everyone in organic chemistry is interested in and puzzled by the mechanism of this process.”
The authors designed C–N coupling experiments using both stoichiometric and catalytic amounts of a copper-carbazolide complex and exposure at room temperature to a 13-W compact fluorescent lightbulb or a 100-W mercury lamp. Their results indicate that photons cause the copper complex to transfer an electron to the aryl halide, cleaving the aryl halide’s carbon-halogen bond and producing a radical ion pair, which then leads to C–N bond formation between the aryl halide and carbazole. An alternative pathway suggested for non-photoinduced Ullmann couplings had been that carbon-halide bond breaking occurs during a concerted addition to the copper.
“We’re already at work trying to explore how broad the scope of this type of chemistry is,” Peters says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter