Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Velcro-Like Protein Both Activates And Tracks Biochemical Targets
Fluorescent method to control and monitor protein activity is adaptable for diverse proteins
by Carmen Drahl
November 12, 2012
| A version of this story appeared in
Volume 90, Issue 46
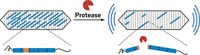
With an engineered protein, researchers have developed an integrated way to control enzyme activity and track it via fluorescence (Science, DOI: 10.1126/science.1226854). Manipulating proteins with light is nothing new—scientists have used both small molecules and other proteins to take control. The existing approaches, however, are difficult to generalize to diverse proteins. Xin X. Zhou, Michael Z. Lin, and coworkers at Stanford University have now adapted Dronpa, a fluorescent protein from coral, to the task. Left alone, their glowing Dronpa variants stick to one another like Velcro. Under blue-green light (about 500 nm), the dimers or tetramers fall apart, dimming in fluorescence. Lin’s team tacked a Dronpa unit to each end of the proteins they wished to study, including a hepatitis C protease and a protein linked to cell locomotion. The Dronpas stuck together, caging the proteins and inactivating them. Blue-green light released the cage, restoring protein activity. The researchers tracked activation through the proteins’ fading fluorescence. Lin’s lab is refining the technology so that it will work with any part of a protein, not just the ends. Stanford has filed a patent application on the technique.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter